Mechanisms involved in controlling RNA virus-induced intestinal inflammation
- PMID: 35604464
- PMCID: PMC9125963
- DOI: 10.1007/s00018-022-04332-z
Mechanisms involved in controlling RNA virus-induced intestinal inflammation
Abstract
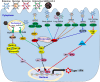
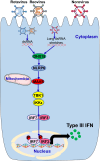
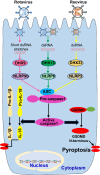
Gastroenteritis is inflammation of the lining of stomach and intestines and causes significant morbidity and mortality worldwide. Many viruses, especially RNA viruses are the most common cause of enteritis. Innate immunity is the first line of host defense against enteric RNA viruses and virus-induced intestinal inflammation. The first layer of defense against enteric RNA viruses in the intestinal tract is intestinal epithelial cells (IECs), dendritic cells and macrophages under the intestinal epithelium. These innate immune cells express pathogen-recognition receptors (PRRs) for recognizing enteric RNA viruses through sensing viral pathogen-associated molecular patterns (PAMPs). As a result of this recognition type I interferon (IFN), type III IFN and inflammasome activation occurs, which function cooperatively to clear infection and reduce viral-induced intestinal inflammation. In this review, we summarize recent findings about mechanisms involved in enteric RNA virus-induced intestinal inflammation. We will provide an overview of the enteric RNA viruses, their RNA sensing mechanisms by host PRRs, and signaling pathways triggered by host PRRs, which shape the intestinal immune response to maintain intestinal homeostasis.
Keywords: Caspase; Cytokine; Diarrhea; Enterocyte; PARP9; Pyroptosis; RNA helicase; SARS-CoV-2.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Black RE, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

