Insulin-like growth factor I sensitization rejuvenates sleep patterns in old mice
- PMID: 35604612
- PMCID: PMC9616996
- DOI: 10.1007/s11357-022-00589-1
Insulin-like growth factor I sensitization rejuvenates sleep patterns in old mice
Abstract
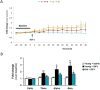
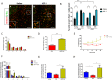
Sleep disturbances are common during aging. Compared to young animals, old mice show altered sleep structure, with changes in both slow and fast electrocorticographic (ECoG) activity and fewer transitions between sleep and wake stages. Insulin-like growth factor I (IGF-I), which is involved in adaptive changes during aging, was previously shown to increase ECoG activity in young mice and monkeys. Furthermore, IGF-I shapes sleep architecture by modulating the activity of mouse orexin neurons in the lateral hypothalamus (LH). We now report that both ECoG activation and excitation of orexin neurons by systemic IGF-I are abrogated in old mice. Moreover, orthodromical responses of LH neurons are facilitated by either systemic or local IGF-I in young mice, but not in old ones. As orexin neurons of old mice show dysregulated IGF-I receptor (IGF-IR) expression, suggesting disturbed IGF-I sensitivity, we treated old mice with AIK3a305, a novel IGF-IR sensitizer, and observed restored responses to IGF-I and rejuvenation of sleep patterns. Thus, disturbed sleep structure in aging mice may be related to impaired IGF-I signaling onto orexin neurons, reflecting a broader loss of IGF-I activity in the aged mouse brain.
Keywords: Aging; Cortical activation; IGF-I; Orexinergic neurons; Rejuvenation; Sleep.
© 2022. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
MV and ITA have shares in Allinky BioPharma, manufacturer of AIK3a.
Figures




Similar articles
-
Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons.FASEB J. 2020 Dec;34(12):15975-15990. doi: 10.1096/fj.202001281RR. Epub 2020 Oct 8. FASEB J. 2020. PMID: 33070417
-
Insulin-like Growth Factor I Couples Metabolism with Circadian Activity through Hypothalamic Orexin Neurons.Int J Mol Sci. 2022 Apr 23;23(9):4679. doi: 10.3390/ijms23094679. Int J Mol Sci. 2022. PMID: 35563069 Free PMC article.
-
Insulin-like growth factor I mitigates post-traumatic stress by inhibiting AMP-kinase in orexin neurons.Mol Psychiatry. 2022 Apr;27(4):2182-2196. doi: 10.1038/s41380-022-01442-9. Epub 2022 Feb 3. Mol Psychiatry. 2022. PMID: 35115701 Free PMC article.
-
Local network regulation of orexin neurons in the lateral hypothalamus.Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R572-80. doi: 10.1152/ajpregu.00674.2010. Epub 2011 Jun 22. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21697524 Review.
-
Sleep-wake and arousal dysfunctions in post-traumatic stress disorder: Role of orexin systems.Brain Res Bull. 2022 Aug;186:106-122. doi: 10.1016/j.brainresbull.2022.05.006. Epub 2022 May 23. Brain Res Bull. 2022. PMID: 35618150 Review.
Cited by
-
The signaling landscape of insulin-like growth factor 1.J Biol Chem. 2025 Jan;301(1):108047. doi: 10.1016/j.jbc.2024.108047. Epub 2024 Dec 3. J Biol Chem. 2025. PMID: 39638246 Free PMC article. Review.
-
The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer's Disease.Int J Mol Sci. 2023 Nov 17;24(22):16440. doi: 10.3390/ijms242216440. Int J Mol Sci. 2023. PMID: 38003628 Free PMC article. Review.
-
Insulin-like Growth Factor 1 Impact on Alzheimer's Disease: Role in Inflammation, Stress, and Cognition.Curr Issues Mol Biol. 2025 Mar 27;47(4):233. doi: 10.3390/cimb47040233. Curr Issues Mol Biol. 2025. PMID: 40699632 Free PMC article. Review.
-
Untangling IGF-I signaling in the aging brain.Aging (Albany NY). 2023 Feb 3;15(3):599-600. doi: 10.18632/aging.204507. Epub 2023 Feb 3. Aging (Albany NY). 2023. PMID: 36734934 Free PMC article. No abstract available.
-
Endothelial deficiency of insulin-like growth factor-1 receptor leads to blood-brain barrier disruption and accelerated endothelial senescence in mice, mimicking aspects of the brain aging phenotype.Microcirculation. 2024 Feb;31(2):e12840. doi: 10.1111/micc.12840. Epub 2023 Dec 11. Microcirculation. 2024. PMID: 38082450 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

