Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study
- PMID: 35605188
- PMCID: PMC9622589
- DOI: 10.1200/JCO.21.02278
Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study
Abstract
Purpose: Lorlatinib significantly improved progression-free survival (PFS) versus crizotinib and showed robust intracranial activity in patients with previously untreated advanced ALK-positive non-small-cell lung cancer (NSCLC) in the phase III CROWN trial. Here, we report post hoc efficacy outcomes in patients with and without brain metastases at baseline, and present data on the incidence and management of CNS adverse events (AEs) in CROWN.
Methods: Eligible patients were randomly assigned 1:1 to first-line lorlatinib (100 mg once daily) or crizotinib (250 mg twice a day); no crossover between treatment arms was permitted. Tumor assessments, including CNS magnetic resonance imaging, were performed at screening and then at 8-week intervals. Regular assessments of patient-reported outcomes were conducted.
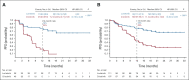
Results: PFS by blinded independent central review was improved with lorlatinib versus crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% v 22% and 78% v 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression versus crizotinib in patients with (7% v 72%) and without (1% v 18%) brain metastases at baseline. In total, 35% of patients had CNS AEs with lorlatinib, most of grade 1 severity. Occurrence of CNS AEs did not result in a clinically meaningful difference in patient-reported quality of life. At analysis, 56% of CNS AEs had resolved (33% without intervention; 17% with lorlatinib dose modification), and 38% were unresolved; most required no intervention. Lorlatinib dose modification did not notably influence PFS.
Conclusion: First-line lorlatinib improved PFS outcomes and reduced CNS progression versus crizotinib in patients with advanced ALK-positive non-small-cell lung cancer with or without brain metastases at baseline. Half of all CNS AEs resolved without intervention or with lorlatinib dose modification.
Trial registration: ClinicalTrials.gov NCT03052608.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures







Comment in
-
Lorlatinib in Frontline Therapy for ALK+ Advanced Non-Small-Cell Lung Cancer: Still a Matter of Debate?J Clin Oncol. 2022 Nov 1;40(31):3564-3568. doi: 10.1200/JCO.22.00859. Epub 2022 Jun 9. J Clin Oncol. 2022. PMID: 35679525 No abstract available.
References
-
- Pfizer Inc : LORBRENA® (lorlatinib): Prescribing Information. http://labeling.pfizer.com/ShowLabeling.aspx?id=11140
-
- Shaw AT, Bauer TM, de Marinis F, et al. : First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383:2018-2029, 2020 - PubMed
-
- Peters S, Camidge DR, Shaw AT, et al. : Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829-838, 2017 - PubMed
-
- Camidge DR, Kim HR, Ahn M-J, et al. : Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 379:2027-2039, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

