Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile
- PMID: 35605637
- PMCID: PMC9307483
- DOI: 10.1038/s41591-022-01874-4
Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile
Abstract
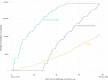
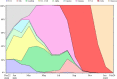
The outbreak of the B.1.1.529 lineage of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Omicron) has caused an unprecedented number of Coronavirus Disease 2019 (COVID-19) cases, including pediatric hospital admissions. Policymakers urgently need evidence of vaccine effectiveness in children to balance the costs and benefits of vaccination campaigns, but, to date, the evidence is sparse. Leveraging a population-based cohort in Chile of 490,694 children aged 3-5 years, we estimated the effectiveness of administering a two-dose schedule, 28 days apart, of Sinovac's inactivated SARS-CoV-2 vaccine (CoronaVac). We used inverse probability-weighted survival regression models to estimate hazard ratios of symptomatic COVID-19, hospitalization and admission to an intensive care unit (ICU) for children with complete immunization over non-vaccination, accounting for time-varying vaccination exposure and relevant confounders. The study was conducted between 6 December 2021 and 26 February 2022, during the Omicron outbreak in Chile. The estimated vaccine effectiveness was 38.2% (95% confidence interval (CI), 36.5-39.9) against symptomatic COVID-19, 64.6% (95% CI, 49.6-75.2) against hospitalization and 69.0% (95% CI, 18.6-88.2) against ICU admission. The effectiveness against symptomatic COVID-19 was modest; however, protection against severe disease was high. These results support vaccination of children aged 3-5 years to prevent severe illness and associated complications and highlight the importance of maintaining layered protections against SARS-CoV-2 infection.
© 2022. The Author(s).
Conflict of interest statement
R.A. participated in an online, international advisory board organized by AstraZeneca on 21 March 2022.
Figures

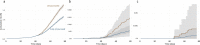
Similar articles
-
Effectiveness of an inactivated SARS-CoV-2 vaccine in children and adolescents: a large-scale observational study.Lancet Reg Health Am. 2023 May;21:100487. doi: 10.1016/j.lana.2023.100487. Epub 2023 Apr 20. Lancet Reg Health Am. 2023. PMID: 37155483 Free PMC article.
-
Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study.Lancet Glob Health. 2022 Jun;10(6):e798-e806. doi: 10.1016/S2214-109X(22)00112-7. Epub 2022 Apr 23. Lancet Glob Health. 2022. PMID: 35472300 Free PMC article.
-
Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile.N Engl J Med. 2021 Sep 2;385(10):875-884. doi: 10.1056/NEJMoa2107715. Epub 2021 Jul 7. N Engl J Med. 2021. PMID: 34233097 Free PMC article.
-
Real-world effectiveness and factors associated with effectiveness of inactivated SARS-CoV-2 vaccines: a systematic review and meta-regression analysis.BMC Med. 2023 Apr 27;21(1):160. doi: 10.1186/s12916-023-02861-3. BMC Med. 2023. PMID: 37106390 Free PMC article.
-
The effect of COVID-19 vaccine to the Omicron variant in children and adolescents: a systematic review and meta-analysis.Front Public Health. 2024 Apr 10;12:1338208. doi: 10.3389/fpubh.2024.1338208. eCollection 2024. Front Public Health. 2024. PMID: 38660347 Free PMC article.
Cited by
-
Household transmission of SARS-CoV-2 during the Omicron wave in Shanghai, China: A case-ascertained study.Influenza Other Respir Viruses. 2023 Feb;17(2):e13097. doi: 10.1111/irv.13097. Influenza Other Respir Viruses. 2023. PMID: 36843225 Free PMC article.
-
Epidemiological characteristics of paediatric Omicron infection during the outbreak of SARS-CoV-2 infection during March-May in 2022 in Shanghai, China.Epidemiol Infect. 2023 May 5;151:e81. doi: 10.1017/S0950268823000663. Epidemiol Infect. 2023. PMID: 37142552 Free PMC article.
-
Narrative Review of the Evolution of COVID-19 Vaccination Recommendations in Countries in Latin America, Africa and the Middle East, and Asia.Infect Dis Ther. 2023 May;12(5):1237-1264. doi: 10.1007/s40121-023-00804-2. Epub 2023 Apr 25. Infect Dis Ther. 2023. PMID: 37097556 Free PMC article. Review.
-
Clinical and psychological status analysis of children and parents infected with familial aggregation omicron variant in Shanghai in parent-child ward.Heliyon. 2022 Dec 9;8(12):e12151. doi: 10.1016/j.heliyon.2022.e12151. eCollection 2022 Dec. Heliyon. 2022. PMID: 36578400 Free PMC article.
-
Government inaction on COVID-19 vaccines contributes to the persistence of childism in Brazil.Lancet Reg Health Am. 2022 Sep;13:100346. doi: 10.1016/j.lana.2022.100346. Epub 2022 Aug 7. Lancet Reg Health Am. 2022. PMID: 35966820 Free PMC article. No abstract available.
References
-
- World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

