Inhibition of D-2HG leads to upregulation of a proinflammatory gene signature in a novel HLA-A2/HLA-DR1 transgenic mouse model of IDH1R132H-expressing glioma
- PMID: 35606087
- PMCID: PMC9174833
- DOI: 10.1136/jitc-2022-004644
Inhibition of D-2HG leads to upregulation of a proinflammatory gene signature in a novel HLA-A2/HLA-DR1 transgenic mouse model of IDH1R132H-expressing glioma
Abstract
Background: Long-term prognosis of WHO grade II, isocitrate dehydrogenase (IDH)-mutated low-grade glioma (LGG) is poor due to high risks of recurrence and malignant transformation into high-grade glioma. Immunotherapy strategies are attractive given the relatively intact immune system of patients with LGG and the slow tumor growth rate. However, accumulation of the oncometabolite D-2-hydroxyglutarate (D-2HG) in IDH-mutated gliomas leads to suppression of inflammatory pathways in the tumor microenvironment, thereby contributing to the 'cold' tumor phenotype. Inhibiting D-2HG production presents an opportunity to generate a robust antitumor response following tumor antigen vaccination and immune checkpoint blockade.
Methods: An IDH1R132H glioma model was created in syngeneic HLA-A2/HLA-DR1-transgenic mice, allowing us to evaluate the vaccination with the human leukocyte antigens (HLA)-DR1-restricted, IDH1R132H mutation-derived neoepitope. The effects of an orally available inhibitor of mutant IDH1 and IDH2, AG-881, were evaluated as monotherapy and in combination with the IDH1R132H peptide vaccination or anti-PD-1 immune checkpoint blockade.
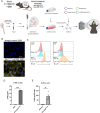
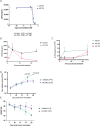
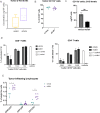
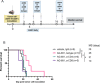
Results: The HLA-A2/HLA-DR1-syngeneic IDH1R132H cell line expressed the IDH1 mutant protein and formed D-2HG producing orthotopic gliomas in vivo. Treatment of tumor-bearing mice with AG-881 resulted in a reduction of D-2HG levels in IDH1R132H glioma cells (10 fold) and tumor-associated myeloid cells, which demonstrated high levels of intracellular D-2HG in the IDH1R132H gliomas. AG-881 monotherapy suppressed the progression of IDH1R132H gliomas in a CD4+ and CD8+ cell-dependent manner, enhanced proinflammatory IFNγ-related gene expression, and increased the number of CD4+ tumor-infiltrating T-cells. Prophylactic vaccination with the HLA-DR1-restricted IDH1R132H peptide or tumor-associated HLA-A2-restricted peptides did not enhance survival of tumor-bearing animals; however, vaccination with both HLA-A2-IDH1R132H and DR1-IDH1R132H peptides in combination with the IDH inhibitor significantly prolonged survival. Finally, tumor-bearing mice treated with both AG-881 and a PD-1 blocking antibody demonstrated improved survival when compared with either treatment alone.
Conclusion: The development of effective IDH1R132H-targeting vaccine may be enhanced by integration with HLA class I-restricted cytotoxic T cell epitopes and AG-881. Our HLA-A2/HLA-DR1-syngeneic IDH1R132H glioma model should allow us to evaluate key translational questions related to the development of novel strategies for patients with IDH-mutant glioma.
Keywords: Brain Neoplasms; Central Nervous System Neoplasms; Drug Evaluation, Preclinical; Programmed Cell Death 1 Receptor; Vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CH, RN, SR, AET, MLH, and BN are employees of Agios (currently) Servier Pharmaceuticals, Boston, MA.
Figures

Similar articles
-
Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice.J Clin Invest. 2021 Feb 15;131(4):e139542. doi: 10.1172/JCI139542. J Clin Invest. 2021. PMID: 33332283 Free PMC article.
-
A vaccine targeting mutant IDH1 induces antitumour immunity.Nature. 2014 Aug 21;512(7514):324-7. doi: 10.1038/nature13387. Epub 2014 Jun 25. Nature. 2014. PMID: 25043048
-
IDH1 p.R132H ctDNA and D-2-hydroxyglutarate as CSF biomarkers in patients with IDH-mutant gliomas.J Neurooncol. 2022 Sep;159(2):261-270. doi: 10.1007/s11060-022-04060-1. Epub 2022 Jul 11. J Neurooncol. 2022. PMID: 35816267 Free PMC article.
-
Consequences of IDH1/2 Mutations in Gliomas and an Assessment of Inhibitors Targeting Mutated IDH Proteins.Molecules. 2019 Mar 9;24(5):968. doi: 10.3390/molecules24050968. Molecules. 2019. PMID: 30857299 Free PMC article. Review.
-
ML309: A potent inhibitor of R132H mutant IDH1 capable of reducing 2-hydroxyglutarate production in U87 MG glioblastoma cells.2012 Apr 16 [updated 2013 May 8]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2012 Apr 16 [updated 2013 May 8]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23905201 Free Books & Documents. Review.
Cited by
-
Oncometabolite 2-hydroxyglutarate regulates anti-tumor immunity.Heliyon. 2024 Jan 10;10(2):e24454. doi: 10.1016/j.heliyon.2024.e24454. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293535 Free PMC article. Review.
-
Effects of metabolic cancer therapy on tumor microenvironment.Front Oncol. 2022 Dec 13;12:1046630. doi: 10.3389/fonc.2022.1046630. eCollection 2022. Front Oncol. 2022. PMID: 36582801 Free PMC article. Review.
-
Exploring the Regulatory Mechanism of CXCL16 Molecule-Related Antigen Presentation Using lncRNA-mRNA Co-Expression Network Analysis.J Inflamm Res. 2024 Dec 24;17:11561-11575. doi: 10.2147/JIR.S496133. eCollection 2024. J Inflamm Res. 2024. PMID: 39735899 Free PMC article.
-
Glioma-neuronal circuit remodeling induces regional immunosuppression.Nat Commun. 2025 May 22;16(1):4770. doi: 10.1038/s41467-025-60074-z. Nat Commun. 2025. PMID: 40404658 Free PMC article.
-
New insights into the Immune TME of adult-type diffuse gliomas.Curr Opin Neurol. 2022 Dec 1;35(6):794-802. doi: 10.1097/WCO.0000000000001112. Epub 2022 Oct 7. Curr Opin Neurol. 2022. PMID: 36226710 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
