ADAMTS4-specific MR probe to assess aortic aneurysms in vivo using synthetic peptide libraries
- PMID: 35606349
- PMCID: PMC9126943
- DOI: 10.1038/s41467-022-30464-8
ADAMTS4-specific MR probe to assess aortic aneurysms in vivo using synthetic peptide libraries
Abstract
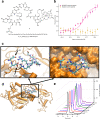
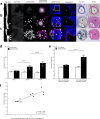
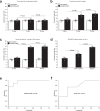
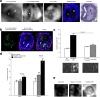
The incidence of abdominal aortic aneurysms (AAAs) has substantially increased during the last 20 years and their rupture remains the third most common cause of sudden death in the cardiovascular field after myocardial infarction and stroke. The only established clinical parameter to assess AAAs is based on the aneurysm size. Novel biomarkers are needed to improve the assessment of the risk of rupture. ADAMTS4 (A Disintegrin And Metalloproteinase with ThromboSpondin motifs 4) is a strongly upregulated proteoglycan cleaving enzyme in the unstable course of AAAs. In the screening of a one-bead-one-compound library against ADAMTS4, a low-molecular-weight cyclic peptide is discovered with favorable properties for in vivo molecular magnetic resonance imaging applications. After identification and characterization, it's potential is evaluated in an AAA mouse model. The ADAMTS4-specific probe enables the in vivo imaging-based prediction of aneurysm expansion and rupture.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

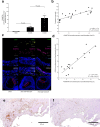
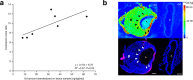
References
-
- Johnston KW, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, Ad Hoc committee on reporting standards, society for vascular surgery and north American chapter, International society for cardiovascular surgery. J. Vasc. Surg. 1991;13:452–458. doi: 10.1067/mva.1991.26737. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

