The interferon-β/STAT1 axis drives the collective invasion of skin squamous cell carcinoma with sealed intercellular spaces
- PMID: 35606369
- PMCID: PMC9126940
- DOI: 10.1038/s41389-022-00403-9
The interferon-β/STAT1 axis drives the collective invasion of skin squamous cell carcinoma with sealed intercellular spaces
Abstract
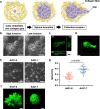
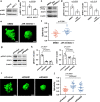
The process by which cancer cells invade as a cell cluster, known as collective invasion, is associated with metastasis and worse prognosis of cancer patients; therefore, inhibition of collective invasion is considered to improve cancer treatment. However, the cellular characteristics responsible for collective invasion remain largely unknown. Here, we successfully established subclones with various invasive potentials derived from human skin squamous carcinoma cells. The cell cluster of the highly invasive subclone had a hermetically sealed and narrow intercellular space. Interferon-β was localized to the sealed intercellular spaces, leading to collective invasion via the activation of signal transducer and activator of transcription 1 (STAT1). On the other hand, interferon-β was not localized to non-sealed and wide intercellular spaces of the cell cluster of low-invasive subclone with deficient STAT1 activity. In the mixed cell cluster of high- and low-invasive subclones, the high-invasive sub-clonal cells were located at the invasive front of the invasive protrusion, leading to collective invasion by the low-invasive sub-clonal cells. Tissue microarray analysis of human skin squamous cell carcinoma (SCC) also showed enrichment of STAT1 in the invasive front of SCCs. These findings indicate that the intercellular structure controls the potential for collective invasion via STAT1 regulation in SCC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The intercellular expression of type-XVII collagen, laminin-332, and integrin-β1 promote contact following during the collective invasion of a cancer cell population.Biochem Biophys Res Commun. 2019 Jul 5;514(4):1115-1121. doi: 10.1016/j.bbrc.2019.05.058. Epub 2019 May 14. Biochem Biophys Res Commun. 2019. PMID: 31101337
-
Collective Polarization of Cancer Cells at the Monolayer Boundary.Micromachines (Basel). 2021 Jan 22;12(2):112. doi: 10.3390/mi12020112. Micromachines (Basel). 2021. PMID: 33499191 Free PMC article.
-
MTMR2 promotes invasion and metastasis of gastric cancer via inactivating IFNγ/STAT1 signaling.J Exp Clin Cancer Res. 2019 May 21;38(1):206. doi: 10.1186/s13046-019-1186-z. J Exp Clin Cancer Res. 2019. PMID: 31113461 Free PMC article.
-
Tactics of cancer invasion: solitary and collective invasion.J Biochem. 2020 Apr 1;167(4):347-355. doi: 10.1093/jb/mvaa003. J Biochem. 2020. PMID: 31926018 Review.
-
The RANKL/RANK system as a therapeutic target for bone invasion by oral squamous cell carcinoma (Review).Int J Oncol. 2013 Mar;42(3):803-9. doi: 10.3892/ijo.2013.1794. Epub 2013 Jan 23. Int J Oncol. 2013. PMID: 23354319 Review.
Cited by
-
Single-Cell Transcriptomics Reveals STAT1 Drives LHPP Downregulation in Esophageal Squamous Cell Carcinoma Progression.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241293485. doi: 10.1177/15330338241293485. Technol Cancer Res Treat. 2024. PMID: 39497541 Free PMC article.
-
Targeting Heme Oxygenase 2 (HO2) with TiNIR, a Theragnostic Approach for Managing Metastatic Non-Small Cell Lung Cancer.Biomater Res. 2024 Apr 25;28:0026. doi: 10.34133/bmr.0026. eCollection 2024. Biomater Res. 2024. PMID: 38665698 Free PMC article.
-
CXCL16 Producing Tumor Clones Are Shaping Immunosuppressive Microenvironment in Squamous Cell Carcinoma via CXCR6 Regulatory T Cell.Cancer Med. 2025 Aug;14(15):e71060. doi: 10.1002/cam4.71060. Cancer Med. 2025. PMID: 40776410 Free PMC article.
References
-
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M et al. SEER Cancer Statistics Review, 1975–2017 (National Cancer Institute, 2020).
-
- Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

