A Fetal Brain magnetic resonance Acquisition Numerical phantom (FaBiAN)
- PMID: 35606398
- PMCID: PMC9127105
- DOI: 10.1038/s41598-022-10335-4
A Fetal Brain magnetic resonance Acquisition Numerical phantom (FaBiAN)
Abstract
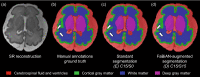
Accurate characterization of in utero human brain maturation is critical as it involves complex and interconnected structural and functional processes that may influence health later in life. Magnetic resonance imaging is a powerful tool to investigate equivocal neurological patterns during fetal development. However, the number of acquisitions of satisfactory quality available in this cohort of sensitive subjects remains scarce, thus hindering the validation of advanced image processing techniques. Numerical phantoms can mitigate these limitations by providing a controlled environment with a known ground truth. In this work, we present FaBiAN, an open-source Fetal Brain magnetic resonance Acquisition Numerical phantom that simulates clinical T2-weighted fast spin echo sequences of the fetal brain. This unique tool is based on a general, flexible and realistic setup that includes stochastic fetal movements, thus providing images of the fetal brain throughout maturation comparable to clinical acquisitions. We demonstrate its value to evaluate the robustness and optimize the accuracy of an algorithm for super-resolution fetal brain magnetic resonance imaging from simulated motion-corrupted 2D low-resolution series compared to a synthetic high-resolution reference volume. We also show that the images generated can complement clinical datasets to support data-intensive deep learning methods for fetal brain tissue segmentation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
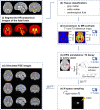
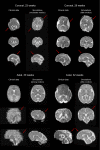


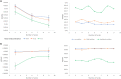
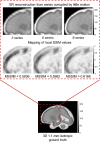
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

