Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine
- PMID: 35606426
- PMCID: PMC9126106
- DOI: 10.1038/s41598-022-12750-z
Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine
Abstract
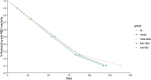
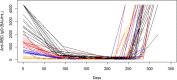
Immunosurveillance by evaluating anti-spike protein receptor-binding domain (S-RBD) antibodies represents a useful tool to estimate the long immunity against Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) infection. The aim of this study was to evaluate the kinetics of antibody response in vaccine recipients. We measured anti-S-RBD IgG levels by indirect chemiluminescence immunoassay on Maglumi 800 (SNIBE, California) in 1013 healthy individuals naïve to SARS-CoV2 infection after two and three COVID-19 vaccine doses. We found that anti-S-RBD IgG levels are higher in females than males. Antibody levels gradually decrease to a steady state after four months since the peak, and the decay is independent of age, sex, vaccine doses, and baseline antibodies titer. The third dose induces a high anti-S-RBD IgG reactivity in individuals with previous high responses and triggers a moderate-high anti-S-RBD IgG reactivity. The assessment of anti-S-RBD IgG levels is essential for monitoring long-term antibody response. A third SARS-CoV-2 vaccine dose is associated with a significant immunological response. Thus, our results support the efficacy of the vaccine programs and the usefulness of the third dose.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Strong Decay of SARS-CoV-2 Spike Antibodies after 2 BNT162b2 Vaccine Doses and High Antibody Response to a Third Dose in Nursing Home Residents.J Am Med Dir Assoc. 2022 May;23(5):750-753. doi: 10.1016/j.jamda.2022.02.006. Epub 2022 Feb 23. J Am Med Dir Assoc. 2022. PMID: 35311651 Free PMC article.
-
Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: could the booster dose be delayed?Infection. 2022 Dec;50(6):1573-1577. doi: 10.1007/s15010-022-01816-9. Epub 2022 Apr 7. Infection. 2022. PMID: 35391649 Free PMC article.
-
Antibody Responses to BNT162b2 Vaccination in Japan: Monitoring Vaccine Efficacy by Measuring IgG Antibodies against the Receptor-Binding Domain of SARS-CoV-2.Microbiol Spectr. 2022 Feb 23;10(1):e0118121. doi: 10.1128/spectrum.01181-21. Epub 2022 Jan 19. Microbiol Spectr. 2022. PMID: 35044205 Free PMC article.
-
Kinetics of anti-SARS-CoV-2 responses post complete vaccination with coronavac: A prospective study in 50 health workers.J Public Health Res. 2022 Aug 9;11(3):22799036221104173. doi: 10.1177/22799036221104173. eCollection 2022 Jul. J Public Health Res. 2022. PMID: 35966047 Free PMC article. Review.
Cited by
-
Incidence and Severity of COVID-19 in Relation to Anti-Receptor-Binding Domain IgG Antibody Level after COVID-19 Vaccination in Kidney Transplant Recipients.Viruses. 2024 Jan 12;16(1):114. doi: 10.3390/v16010114. Viruses. 2024. PMID: 38257814 Free PMC article.
-
Kinetics of Humoral Immunity against SARS-CoV-2 in Healthcare Workers after the Third Dose of BNT162b2 mRNA Vaccine.Vaccines (Basel). 2022 Nov 17;10(11):1948. doi: 10.3390/vaccines10111948. Vaccines (Basel). 2022. PMID: 36423043 Free PMC article.
-
COVID-19 and Frailty.Vaccines (Basel). 2023 Mar 7;11(3):606. doi: 10.3390/vaccines11030606. Vaccines (Basel). 2023. PMID: 36992190 Free PMC article. Review.
-
Immunogenicity evaluation after BNT162b2 booster vaccination in healthcare workers.Sci Rep. 2022 Jul 26;12(1):12716. doi: 10.1038/s41598-022-16759-2. Sci Rep. 2022. PMID: 35882871 Free PMC article.
-
The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers.Viruses. 2023 Jul 18;15(7):1574. doi: 10.3390/v15071574. Viruses. 2023. PMID: 37515259 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

