Virtual exam for Parkinson's disease enables frequent and reliable remote measurements of motor function
- PMID: 35606508
- PMCID: PMC9126938
- DOI: 10.1038/s41746-022-00607-8
Virtual exam for Parkinson's disease enables frequent and reliable remote measurements of motor function
Erratum in
-
Author Correction: Virtual exam for Parkinson's disease enables frequent and reliable remote measurements of motor function.NPJ Digit Med. 2022 Dec 28;5(1):196. doi: 10.1038/s41746-022-00744-0. NPJ Digit Med. 2022. PMID: 36577809 Free PMC article. No abstract available.
Abstract
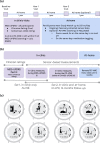
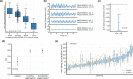
Sensor-based remote monitoring could help better track Parkinson's disease (PD) progression, and measure patients' response to putative disease-modifying therapeutic interventions. To be useful, the remotely-collected measurements should be valid, reliable, and sensitive to change, and people with PD must engage with the technology. We developed a smartwatch-based active assessment that enables unsupervised measurement of motor signs of PD. Participants with early-stage PD (N = 388, 64% men, average age 63) wore a smartwatch for a median of 390 days. Participants performed unsupervised motor tasks both in-clinic (once) and remotely (twice weekly for one year). Dropout rate was 5.4%. Median wear-time was 21.1 h/day, and 59% of per-protocol remote assessments were completed. Analytical validation was established for in-clinic measurements, which showed moderate-to-strong correlations with consensus MDS-UPDRS Part III ratings for rest tremor (⍴ = 0.70), bradykinesia (⍴ = -0.62), and gait (⍴ = -0.46). Test-retest reliability of remote measurements, aggregated monthly, was good-to-excellent (ICC = 0.75-0.96). Remote measurements were sensitive to the known effects of dopaminergic medication (on vs off Cohen's d = 0.19-0.54). Of note, in-clinic assessments often did not reflect the patients' typical status at home. This demonstrates the feasibility of smartwatch-based unsupervised active tests, and establishes the analytical validity of associated digital measurements. Weekly measurements provide a real-life distribution of disease severity, as it fluctuates longitudinally. Sensitivity to medication-induced change and improved reliability imply that these methods could help reduce sample sizes needed to demonstrate a response to therapeutic interventions or disease progression.
© 2022. The Author(s).
Conflict of interest statement
All authors with Verily affiliation are current Verily employees and are Verily shareholders. The study is financially supported by Verily Life Sciences LLC, Radboud University Medical Center, Radboud University, the city of Nijmegen, and the Province of Gelderland. Radboud UMC received funding for the original cohort from Health Holland (PPP allowance; public-private partnership), Radboud University Radboudumc, and Radboudumc (in kind). The other study sponsors had no role in study design and collection, analysis and interpretation of data, or in writing the manuscript. Prof. Bloem serves as the co-Editor in Chief for the Journal of Parkinson’s disease, serves on the editorial board of Practical Neurology and Digital Biomarkers, has received fees from serving on the scientific advisory board for UCB, Kyowa Kirin, Zambon, and the Critical Path Institute (paid to the. Institute), has received fees for speaking at conferences from AbbVie, Biogen, UCB, Zambon, Roche, GE Healthcare, Oruen and Bial (paid to the Institute), and has received research support from the Netherlands Organization for Health Research and Development, the Michael J Fox Foundation, UCB, the Stichting Parkinson Fonds, Hersenstichting Nederland, de Stichting Woelse Waard, Stichting Alkemade-Keuls, de Maag Lever Darm Stichting, Parkinson NL, Davis Phinney Foundation, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, Nothing Impossible and the Parkinson Vereniging, outside the submitted work. Prof. Bloem does not hold any stocks or stock options with any companies that are connected to Parkinson’s disease or to any of the topics in this paper. Prof. Bloem has received grants/research support from Netherlands Organization for Scientific Research, the Michael J Fox Foundation, Nothing Impossible, Parkinson Vereniging, Parkinson’s Foundation, Hersenstichting Nederland, Davis Phinney Foundation, Parkinson NL, Stichting Woelse Waard, Stichting Alkemade-Keuls, Maag Lever Darm Stichting, Verily Life Sciences, Horizon 2020, Topsector Life Sciences and Health, UCB, Zambon. Prof. Bloem has been a consultant for the Critical Path Institute, Kyowa Kirin, UCB, Zambon, and has received speaking fees from AbbVie, Bial, Biogen, GE Healthcare, Oruen, Roche, UCB, Zambon. The remaining authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

