Drug Distribution in Brain and Cerebrospinal Fluids in Relation to IC50 Values in Aging and Alzheimer's Disease, Using the Physiologically Based LeiCNS-PK3.0 Model
- PMID: 35606598
- PMCID: PMC9246802
- DOI: 10.1007/s11095-022-03281-3
Drug Distribution in Brain and Cerebrospinal Fluids in Relation to IC50 Values in Aging and Alzheimer's Disease, Using the Physiologically Based LeiCNS-PK3.0 Model
Abstract
Background: Very little knowledge exists on the impact of Alzheimer's disease on the CNS target site pharmacokinetics (PK).
Aim: To predict the CNS PK of cognitively healthy young and elderly and of Alzheimer's patients using the physiologically based LeiCNS-PK3.0 model.
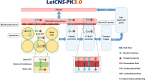
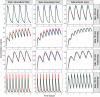
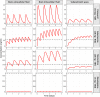
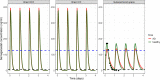
Methods: LeiCNS-PK3.0 was used to predict the PK profiles in brain extracellular (brainECF) and intracellular (brainICF) fluids and cerebrospinal fluid of the subarachnoid space (CSFSAS) of donepezil, galantamine, memantine, rivastigmine, and semagacestat in young, elderly, and Alzheimer's patients. The physiological parameters of LeiCNS-PK3.0 were adapted for aging and Alzheimer's based on an extensive literature search. The CNS PK profiles at plateau for clinical dose regimens were related to in vitro IC50 values of acetylcholinesterase, butyrylcholinesterase, N-methyl-D-aspartate, or gamma-secretase.
Results: The PK profiles of all drugs differed between the CNS compartments regarding plateau levels and fluctuation. BrainECF, brainICF and CSFSAS PK profile relationships were different between the drugs. Aging and Alzheimer's had little to no impact on CNS PK. Rivastigmine acetylcholinesterase IC50 values were not reached. Semagacestat brain PK plateau levels were below the IC50 of gamma-secretase for half of the interdose interval, unlike CSFSAS PK profiles that were consistently above IC50. CONCLUSION: This study provides insights into the relations between CNS compartments PK profiles, including target sites. CSFSAS PK appears to be an unreliable predictor of brain PK. Also, despite extensive changes in blood-brain barrier and brain properties in Alzheimer's, this study shows that the impact of aging and Alzheimer's pathology on CNS distribution of the five drugs is insignificant.
Keywords: Alzheimer’s; aging; physiologically based pharmacokinetics.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
-
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. - DOI - PubMed
-
- Parsons CG, Gilling KE, Jatzke C. Memantine does not show intracellular block of the NMDA receptor channel. Eur J Pharmacol 2008;587(1–3):99–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

