Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction
- PMID: 35606759
- PMCID: PMC9125910
- DOI: 10.1186/s12958-022-00953-y
Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction
Abstract
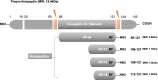
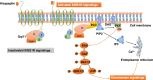
Background: Kisspeptin is the leading upstream regulator of pulsatile and surge Gonadotrophin-Releasing Hormone secretion (GnRH) in the hypothalamus, which acts as the key governor of the hypothalamic-pituitary-ovary axis.
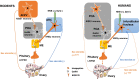
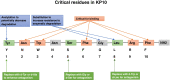
Main text: Exogenous kisspeptin or its receptor agonist can stimulate GnRH release and subsequent physiological gonadotropin secretion in humans. Based on the role of kisspeptin in the hypothalamus, a broad application of kisspeptin and its receptor agonist has been recently uncovered in humans, including central control of ovulation, oocyte maturation (particularly in women at a high risk of ovarian hyperstimulation syndrome), test for GnRH neuronal function, and gatekeepers of puberty onset. In addition, the kisspeptin analogs, such as TAK-448, showed promising agonistic activity in healthy women as well as in women with hypothalamic amenorrhoea or polycystic ovary syndrome.
Conclusion: More clinical trials should focus on the therapeutic effect of kisspeptin, its receptor agonist and antagonist in women with reproductive disorders, such as hypothalamic amenorrhoea, polycystic ovary syndrome, and endometriosis.
Keywords: Female reproduction; Hypothalamic-pituitary-ovarian axis; Hypothalamus; KISS1R; Kisspeptin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Marques P, Skorupskaite K, George JT, Anderson RA, et al. Physiology of GNRH and Gonadotropin Secretion. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth: MDText.com, Inc. Copyright © 2000–2021, MDText.com, Inc.; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

