Marine-Derived Stichloroside C2 Inhibits Epithelial-Mesenchymal Transition and Induces Apoptosis through the Mitogen-Activated Protein Kinase Signalling Pathway in Triple-Negative Breast Cancer Cells
- PMID: 35607324
- PMCID: PMC9124082
- DOI: 10.1155/2022/6449984
Marine-Derived Stichloroside C2 Inhibits Epithelial-Mesenchymal Transition and Induces Apoptosis through the Mitogen-Activated Protein Kinase Signalling Pathway in Triple-Negative Breast Cancer Cells
Abstract
Background: Triterpenoid saponins from sea cucumbers exhibit significant antitumour, antifungal, and antibacterial activities. However, the associated molecular mechanisms have yet to be elucidated. In this study, we screened and explored the antitumour activity and underlying mechanisms of triterpenoid saponins isolated from Thelenota ananas.
Methods: We isolated and purified sea cucumber saponins, determined their chemical structures, and confirmed their function in vitro. We also screened and explored the antitumour activity and underlying mechanisms of triterpenoid saponins isolated from Thelenota ananas.
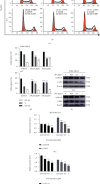
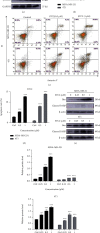
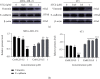
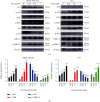
Results: Four saponins were discovered from sea cucumber Thelenota ananas collected from the South China Sea. We found that stichloroside C2 (STC2) inhibited the proliferation and clonogenesis of the human triple-negative breast cancer (TNBC) cell line MDA-MB-231 and mouse TNBC cell line 4 T1 in a dose-dependent manner and induced apoptosis and cycle arrest in these two TNBC cell lines. STC2 induced DNA damage in two TNBC cell lines and significantly increased the protein expression level of the DNA double-strand break marker γ-H2AX. STC2 downregulated the protein expression levels of phosphorylated cyclin-dependent kinase 1 (CDK1), cyclin B1, CDK2, and cyclin A2 in MDA-MB-231 and 4 T1 cells. STC2 upregulated Bax and cleaved PARP protein expression in two types of breast cancer cells. In addition, STC2 promoted E-cadherin expression; inhibited vimentin expression; upregulated the phosphorylation levels of the mitogen-activated protein kinase (MAPK) signalling pathway-related proteins p38, JNK, and ERK1/2; and downregulated Akt phosphorylation.
Conclusions: STC2 exerts anti-TNBC activity, inhibits epithelial-mesenchymal transition (EMT), and induces apoptosis by regulating the cell cycle, EMT-related proteins, and MAPK signalling pathway.
Copyright © 2022 Chuang Cui et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Zheng R. S., Sun K. X., Zhang S. W., et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi . 2019;41(1):19–28. - PubMed
-
- Fu Z. T., Guo X. L., Zhang S. W., et al. Statistical analysis of incidence and mortality of prostate cancer in China, 2015. Zhonghua Zhong Liu Za Zhi . 2020;42(9):718–722. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

