Effects of Ethanolic Extract of Schisandra sphenanthera on the Pharmacokinetics of Rosuvastatin in Rats
- PMID: 35607596
- PMCID: PMC9123906
- DOI: 10.2147/DDDT.S364234
Effects of Ethanolic Extract of Schisandra sphenanthera on the Pharmacokinetics of Rosuvastatin in Rats
Abstract
Purpose: Wuzhi capsule (WZ) is a proprietary Chinese medicine prepared from the ethanolic extract of Schisandra sphenanthera that is commonly used to treat liver injury. Statins are widely used in patients with hyperlipidemia, coronary heart disease, metabolic syndrome, type 2 diabetes mellitus, and nonalcoholic fatty liver disease. Co-administration of statins with WZ is possible in clinical practice. WZ has obvious inhibitory effects on the bioavailability of atorvastatin and simvastatin; however, the drug-herb interactions between WZ and rosuvastatin have not been addressed. We explored the effects of WZ on the pharmacokinetics of rosuvastatin in Sprague-Dawley rats to promote a rational use of statins.
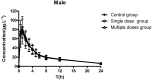
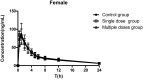
Methods: Eighteen male rats were randomly and evenly divided into three groups: control group (gavage feeding of rosuvastatin 10 mg·kg-1), single dose group (gavage feeding of a single dose of WZ 150 mg·kg-1 followed by rosuvastatin 10 mg·kg-1) and multiple doses group (gavage feeding of WZ 150 mg·kg-1 for 7 days followed by rosuvastatin 10 mg·kg-1 on the seventh day). Plasma samples were collected at different times before and after rosuvastatin administration. The other 18 female rats were tested the same way as the male rats. All samples were analyzed by a validated LC-MS/MS method, and the pharmacokinetic parameters were calculated using a non-compartmental model.
Results: In both male and female rats, there were no statistically significant differences in rosuvastatin pharmacokinetic parameters between the control group, the single dose group, and the multi-dose group.
Conclusion: Acute or long-term intake of WZ had no obvious effect on the pharmacokinetics of rosuvastatin, and therefore rosuvastatin could be used as an alternative to atorvastatin and simvastatin when WZ is clinically required in conjunction with statins. An appropriate pharmacodynamic study is needed to encourage the safe use of this combination.
Keywords: Wuzhi capsule; herb-drug interaction; hyperlipidemia; metabolic syndrome; pharmacokinetics; rosuvastatin.
© 2022 Sun et al.
Conflict of interest statement
The authors report no potential conflicts of interest in this work.
Figures
References
-
- Tabrizi R, Tamtaji OR, Mirhosseini N, et al. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;141:85–103. doi:10.1016/j.phrs.2018.12.010 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

