Prospective F-18 FDOPA PET Imaging Study in Human PD
- PMID: 35607632
- PMCID: PMC9123108
- DOI: 10.1007/s13139-022-00748-4
Prospective F-18 FDOPA PET Imaging Study in Human PD
Abstract
Purpose: We present the findings of our final prospective study submitted to the U.S. Food and Drug Administration (FDA) for New Drug Application (NDA) approval for the use of 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine (F-18 FDOPA) positron emission tomography (PET) imaging for Parkinson's disease (PD). The primary aim was to determine the sensitivity, specificity, and predictive values of F-18 FDOPA PET in parkinsonian patients with respect to clinical standard-of-truth (SOT). Secondary outcomes included the inter-rater reliability, and correlation of quantitative measures for PET with dopaminergic status.
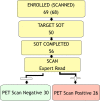
Methods: In 68 parkinsonian subjects, F-18 FDOPA PET scan from 80 to 100 min was acquired following a CT scan. Scan images were presented to one expert in F-18 FDOPA image interpretation and two physicians with prior experience in I-123 FPCIT single-photon emission computed tomography image interpretation. Fifty-six subjects completed the study with a follow-up for SOT determination. Image readers were blind to the clinical/quantitative data; SOT clinician was blind to the image data.
Results: For 47 of the 56 patients, SOT was in agreement with the PET scan results. For nine patients, SOT suggested dopaminergic deficit, whereas the imaging showed normal uptake. The specificity and positive predictive values are 91% and 92%, respectively, suggesting high probability that those who test positive by the PET scan truly have dopaminergic degeneration. The sensitivity was 73%. Inter-rater agreement was 0.6-0.8 between the different readers.
Conclusion: Our prospective study demonstrates high specificity and moderate sensitivity of F-18 FDOPA PET for PD. We received NDA approval in October 2019.
Supplementary information: The online version contains supplementary material available at 10.1007/s13139-022-00748-4.
Keywords: F-18 FDOPA; PET; Parkinsonism; Prospective study.
© The Author(s), under exclusive licence to Korean Society of Nuclear Medicine 2022.
Conflict of interest statement
Competing InterestsVijay Dhawan, Martin H Niethammer, Martin L Lesser, Karalyn N Pappas, Matthew Hellman, Toni M Fitzpatrick, David Bjelke, Jaskirat Singh, Loreta M Quatarolo, Yoon Young Choi, Alice Oh, David Eidelberg, and Thomas Chaly declare that they have no competing financial interests.
Figures


References
-
- Chevalme YM, Montravers F, Vuillez JP, Zanca M, Fallais C, Oustrin J, et al. FDOPA-(18F): a PET radiopharmaceutical recently registered for diagnostic use in countries of the European Union. Braz Arch Biol Tech. 2007;50:77–90. doi: 10.1590/S1516-89132007000600009. - DOI
-
- Calabria FF, Calabria E, Gangemi V, Cascini GL. Current status and future challenges of brain imaging with (18)F-DOPA PET for movement disorders. Hell J Nucl Med. 19:33–41. - PubMed
LinkOut - more resources
Full Text Sources
