Humoral and T-Cell Immune Response After 3 Doses of Messenger RNA Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines in Fragile Patients: The Italian VAX4FRAIL Study
- PMID: 35607769
- PMCID: PMC9213871
- DOI: 10.1093/cid/ciac404
Humoral and T-Cell Immune Response After 3 Doses of Messenger RNA Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines in Fragile Patients: The Italian VAX4FRAIL Study
Abstract
Background: Patients with solid or hematological tumors or neurological and immune-inflammatory disorders are potentially fragile subjects at increased risk of experiencing severe coronavirus disease 2019 and an inadequate response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.
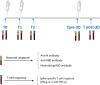
Methods: We designed a prospective Italian multicenter study to assess humoral and T-cell responses to SARS-CoV-2 vaccination in patients (n = 378) with solid tumors (ST), hematological malignancies (HM), neurological disorders (ND), and immunorheumatological diseases (ID). A group of healthy controls was also included. We analyzed the immunogenicity of the primary vaccination schedule and booster dose.
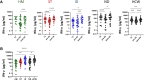
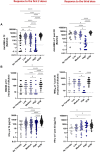
Results: The overall seroconversion rate in patients after 2 doses was 62.1%. Significantly lower rates were observed in HM (52.4%) and ID (51.9%) than in ST (95.6%) and ND (70.7%); a lower median antibody level was detected in HM and ID versus ST and ND (P < .0001). Similar rates of patients with a positive SARS-CoV-2 T-cell response were found in all disease groups, with a higher level observed in ND. The booster dose improved the humoral response in all disease groups, although to a lesser extent in HM patients, whereas the T-cell response increased similarly in all groups. In the multivariable logistic model, independent predictors of seroconversion were disease subgroup, treatment type, and age. Ongoing treatment known to affect the immune system was associated with the worst humoral response to vaccination (P < .0001) but had no effect on T-cell responses.
Conclusions: Immunosuppressive treatment more than disease type per se is a risk factor for a low humoral response after vaccination. The booster dose can improve both humoral and T-cell responses.
Keywords: Fragile patients; SARS-CoV-2 mRNA vaccine; T-cell immunity; humoral immunity.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . P. C. reports consulting fees for advisory board participation from AbbVie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, and Takeda; payment or honoraria for lectures from AbbVie, Amgen, Celgene, Gilead/Kite, Janssen, Novartis, Roche, Sanofi, Takeda; support for attending meetings and/or travel from AbbVie, Amgen, BMS, Celgene, Gilead/Kite, Janssen, Novartis, Roche, and Takeda. A. Mantovani reports royalties for reagents related to innate immunity; consulting fees and payment or honoraria as a consultant/advisory board member for Novartis, Roche, Ventana, Pierre Fabre, Verily, AbbVie, BMS, J&J, Imcheck, Myeloid Therapeutics, Astra Zeneca, Biovelocita, BG Fund, Third Rock Venture, Violend Verseau Therapeutics, Macrophage pharma, Ellipses Pharma, and Olatec Therapeutics; and is the inventor of patents related to PTX3 and other innate immunity molecules. D. G. reports payment or honoraria for lesson to a Master from Vivamed s.r.l., consulting from MSD Italia, and consulting from MITT Medical and Scientific Learning. PLZ reports consulting fees from Takeda, Janssen, BMS, MSD, Kyowa Kirin, Sanofi, Eusa Pharma, and Roche; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda, MSD, Kyowa Kirin, Sanofi, Beigene, and Roche. R. M. reports consulting fees paid to author from Alexion, Argenx, and UCB; payment to author for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alexion, Argenx, Merck Serono, Reflexion Medical Network, Sanofi Aventis, UCB; paid participation on Data Safety Monitoring or Advisory Board with Alexion, Argenx, Catalyst, and UCB. A. U. reports grants or contracts unrelated to this work from FISM, ALEXION, BIOGEN, ROCHE, MERCK SERONO, and COVAXIMS; participation on Data Safety Monitoring or Advisory Board for BD, BIOGEN, IQVIA, SANOFI, ROCHE, ALEXION, BRISTOL MYERS SQUIBB. N. S. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Lilly, Roche, and Servier. F. L. reports a position as President of the Italian Higher Council of Health, the technical scientific advisory body to the Ministry of Health since 2019 and a position as Coordinator of the Technical-Scientific Committee for the COVID-19 pandemic from March 2021 to March 2022. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Salvarani C, Bajocchi G, Mancuso P, et al. Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: a population-based study. Ann Rheum Dis 2020; 79:986.2–988. Available at:https://ard.bmj.com/lookup/doi/10.1136/annrheumdis-2020-217903. - DOI - PubMed
-
- Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020; 139:43–50. Available at:https://linkinghub.elsevier.com/retrieve/pii/S0959804920304627. - PMC - PubMed
-
- Laroni A, Schiavetti I, Sormani MP, Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler J 2020; 27:2126–36. - PubMed
-
- El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. Available at:https://www.nejm.org/doi/10.1056/NEJMoa2113017. Accessed 29 December 2021. - DOI - PMC - PubMed

