Duration of Replication-Competent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Shedding Among Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19)
- PMID: 35607802
- PMCID: PMC9213867
- DOI: 10.1093/cid/ciac405
Duration of Replication-Competent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Shedding Among Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19)
Abstract
Background: Patterns of shedding replication-competent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in severe or critical COVID-19 are not well characterized. We investigated the duration of replication-competent SARS-CoV-2 shedding in upper and lower airway specimens from patients with severe or critical coronavirus disease 2019 (COVID-19).
Methods: We enrolled patients with active or recent severe or critical COVID-19 who were admitted to a tertiary care hospital intensive care unit (ICU) or long-term acute care hospital (LTACH) because of COVID-19. Respiratory specimens were collected at predefined intervals and tested for SARS-CoV-2 using viral culture and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Clinical and epidemiologic metadata were reviewed.
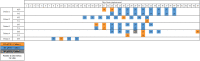
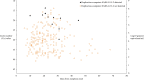
Results: We collected 529 respiratory specimens from 78 patients. Replication-competent virus was detected in 4 of 11 (36.3%) immunocompromised patients up to 45 days after symptom onset and in 1 of 67 (1.5%) immunocompetent patients 10 days after symptom onset (P = .001). All culture-positive patients were in the ICU cohort and had persistent or recurrent symptoms of COVID-19. Median time from symptom onset to first specimen collection was 15 days (range, 6-45) for ICU patients and 58.5 days (range, 34-139) for LTACH patients. SARS-CoV-2 RNA was detected in 40 of 50 (80%) ICU patients and 7 of 28 (25%) LTACH patients.
Conclusions: Immunocompromise and persistent or recurrent symptoms were associated with shedding of replication-competent SARS-CoV-2, supporting the need for improving respiratory symptoms in addition to time as criteria for discontinuation of transmission-based precautions. Our results suggest that the period of potential infectiousness among immunocompetent patients with severe or critical COVID-19 may be similar to that reported for patients with milder disease.
Keywords: SARS-CoV-2; intensive care; long-term acute care hospital; viral culture; virus shedding.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. M. K. H. is a member of a clinical adjudication panel for a SARS-CoV-2 vaccine under development by Sanofi; reports support unrelated to this work from the CDC; and is 2021 President of the SHEA Board of Directors, Chair of IDSA Rapid Guidelines Committee for COVID-19 Diagnosis, and Chair of IDSA Diagnostics Committee. N. M. M. reports a research contract unrelated to this work and paid to his institution from Cepheid, Inc; honoraria for a continuing education lecture from South Central Association for Clinical Microbiology, honoraria for book chapters from Elsevier, honoraria for a continuing education lecture from the American Society for Clinical Laboratory Science; and a position as President-Elect of the Illinois Society for Microbiology. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Centers for Disease Control and Prevention . Duration of isolation and precautions for adults with COVID-19. Centers for Disease Control and Prevention 2020; 2019:1–7. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 29 August 2021.
-
- European Centre for Disease Prevention and Control . Guidance for discharge and ending of isolation of people with COVID-19. 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-for-di.... Accessed 29 August 2021.
-
- World Health Organization . COVID-19 clinical management: living guidance. 2021. Available at:https://apps.who.int/iris/handle/10665/338882. Accessed 29 August 2021.
-
- National Library of Medicine, National Center for Biotechnology Information . NIH coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.ncbi.nlm.nih.gov/books/NBK570371/. Accessed 24 March 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

