How do we…form and coordinate a national serosurvey of SARS-CoV-2 within the blood collection industry?
- PMID: 35607854
- PMCID: PMC9348230
- DOI: 10.1111/trf.16943
How do we…form and coordinate a national serosurvey of SARS-CoV-2 within the blood collection industry?
Abstract
Background: A national serosurvey of U.S. blood donors conducted in partnership with the Centers for Disease Control and Prevention (CDC) was initiated to estimate the prevalence of SARS-CoV-2 infections and vaccinations.
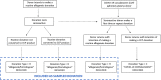
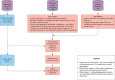
Methods: Beginning in July 2020, the Nationwide Blood Donor Seroprevalence Study collaborated with multiple blood collection organizations, testing labs, and leadership from government partners to capture, test, and analyze approximately 150,000 blood donation specimens per month in a repeated, cross-sectional seroprevalence survey.
Results: A CDC website (https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence) provided stratified, population-level results to public health professionals and the general public.
Discussion: The study adapted operations as the pandemic evolved, changing specimen flow and testing algorithms, and collecting additional data elements in response to changing policies on universal blood donation screening and administration of SARS-CoV-2 spike-based vaccines. The national serosurvey demonstrated the utility of serosurveillance testing of residual blood donations and highlighted the role of the blood collection industry in public-private partnerships during a public health emergency.
© 2022 AABB. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

