Detection of Bourbon Virus-Specific Serum Neutralizing Antibodies in Human Serum in Missouri, USA
- PMID: 35607948
- PMCID: PMC9241549
- DOI: 10.1128/msphere.00164-22
Detection of Bourbon Virus-Specific Serum Neutralizing Antibodies in Human Serum in Missouri, USA
Abstract
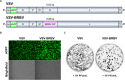
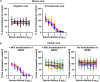
Bourbon virus (BRBV) was first discovered in 2014 in a fatal human case. Since then it has been detected in the tick Amblyomma americanum in the states of Missouri and Kansas in the United States. Despite the high prevalence of BRBV in ticks in these states, very few human cases have been reported, and the true infection burden of BRBV in the community is unknown. Here, we developed two virus neutralization assays, a vesicular stomatitis virus (VSV)-BRBV pseudotyped rapid assay and a BRBV focus reduction neutralization assay, to assess the seroprevalence of BRBV neutralizing antibodies in human sera collected in 2020 in St. Louis, MO. Of 440 human serum samples tested, three (0.7%) were able to potently neutralize both VSV-BRBV and wild-type BRBV. These findings suggest that human infections with BRBV are more common than previously recognized. IMPORTANCE Since the discovery of the Bourbon virus (BRBV) in 2014, a total of five human cases have been identified, including two fatal cases. BRBV is thought to be transmitted by the lone star tick, which is prevalent in the eastern, southeastern, and midwestern United States. BRBV has been detected in ticks in Missouri and Kansas, and serological evidence suggests that it is also present in North Carolina. However, the true infection burden of BRBV in humans is not known. In the present study, we developed two virus neutralization assays to assess the seroprevalence of BRBV-specific antibodies in human sera collected in 2020 in St. Louis, MO. We found that a small subset of individuals are seropositive for neutralizing antibodies against BRBV. Our data suggest that BRBV infection in humans is more common than previously thought.
Keywords: Bourbon virus; glycoprotein; human serum; monoclonal antibody; seroprevalence; virus neutralization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

