Renin-Angiotensin System Pathway Therapeutics Associated With Improved Outcomes in Males Hospitalized With COVID-19
- PMID: 35607951
- PMCID: PMC9380153
- DOI: 10.1097/CCM.0000000000005589
Renin-Angiotensin System Pathway Therapeutics Associated With Improved Outcomes in Males Hospitalized With COVID-19
Abstract
Objectives: To determine whether angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors are associated with improved outcomes in hospitalized patients with COVID-19 according to sex and to report sex-related differences in renin-angiotensin system (RAS) components.
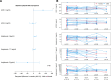
Design: Prospective observational cohort study comparing the effects of ARB or ACE inhibitors versus no ARBs or ACE inhibitors in males versus females. Severe acute respiratory syndrome coronavirus 2 downregulates ACE-2, potentially increasing angiotensin II (a pro-inflammatory vasoconstrictor). Sex-based differences in RAS dysregulation may explain sex-based differences in responses to ARBs because the ACE2 gene is on the X chromosome. We recorded baseline characteristics, comorbidities, prehospital ARBs or ACE inhibitor treatment, use of organ support and mortality, and measured RAS components at admission and days 2, 4, 7, and 14 in a subgroup ( n = 46), recorded d -dimer ( n = 967), comparing males with females.
Setting: ARBs CORONA I is a multicenter Canadian observational cohort of patients hospitalized with acute COVID-19. This analysis includes patients admitted to 10 large urban hospitals across the four most populated provinces.
Patients: One-thousand six-hundred eighty-six patients with polymerase chain reaction-confirmed COVID-19 (February 2020 to March 2021) for acute COVID-19 illness were included.
Interventions: None.
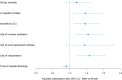
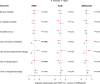
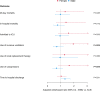
Measurements and main results: Males on ARBs before admission had decreased use of ventilation (adjusted odds ratio [aOR] = 0.52; p = 0.007) and vasopressors (aOR = 0.55; p = 0.011) compared with males not on ARBs or ACE inhibitors. No significant effects were observed in females for these outcomes. The test for interaction was significant for use of ventilation ( p = 0.006) and vasopressors ( p = 0.044) indicating significantly different responses to ARBs according to sex. Males had significantly higher plasma ACE-1 at baseline and angiotensin II at day 7 and 14 than females.
Conclusions: ARBs use was associated with less ventilation and vasopressors in males but not females. Sex-based differences in RAS dysregulation may contribute to sex-based differences in outcomes and responses to ARBs in COVID-19.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Drs. Rocheleau’s, Cheng’s, Lee’s, Haljan’s, Vinh’s, Mann’s, Donohoe’s, and Russell’s institutions received funding from the Canadian Institutes for Health Research. Dr. Mohammed’s institution received funding from Genome Canada and Genome British Colombia. Dr. Goodlett disclosed that he is the founder of Pataigin LLC and Deurion LLC and that the University of Victoria Genome British Columbia Proteome Centre (for which he is a director) received funding from Genome Canada and Genome British Colombia. Dr. Burns disclosed that he is a consultant to JN Nova Pharma. Dr. Cheng’s institution received funding from McGill Interdisciplinary Initiative in Infection and Immunity; he received funding from GEn1E Lifesciences and NPlex Biosciences; he disclosed that he is the cofounder of Kanvas Biosciences and owns equity in the company; and he disclosed that he is a named coinventor on a pending patent entitled “Methods for assessing the severity and progression of SARS-CoV2 infections using cell-free DNA.” Dr. Slutsky received funding from Apeiron, GlaxoSmithKline, Baxter, Cellenkos, Diffusion, Edesa, Exvastat, Faron, and Novalung. Dr. Lee received funding from the Fonds de recherche du Quebec santé (FRQS). Dr. Haljan’s institution received funding from Michael Smith Foundation for Health Research and Surrey Hospital Foundation. Dr. Vinh’s institution received funding from the Jeffrey Modell Foundation and FRQS; he received funding from CSL Behring, Astra Zeneca, Moderna, Takeda, QU Biologics, Merck Canada, Novartis Canada, and the COVID Immunity Task Force (Public Health Agency of Canada); he disclosed he has patent applications pending (Encrypting File System Identification [EFS ID] 40101099; EFS ID 44321620). Dr. Russell reports patents owned by the University of British Columbia (UBC) that are related to the use of proprotein convertase subtilisin/kexin type 9 inhibitor(s) in sepsis and related to the use of vasopressin in septic shock and a patent owned by Ferring for use of selepressin in septic shock; he is an inventor on these patents; he was a founder, director, and shareholder in Cyon Therapeutics and is a shareholder in Molecular You Corp; he reports receiving consulting fees in the last 3 years from: Asahi Kesai Pharmaceuticals of America (was developing recombinant thrombomodulin in sepsis), SIB Therapeutics LLC (developing a sepsis drug), and Ferring Pharmaceuticals (manufactures vasopressin and developing selepressin); he is no longer actively consulting for the following: La Jolla Pharmaceuticals (developing angiotensin II; Dr. Russell chaired the Data Safety Monitoring Board of a trial of angiotensin II from 2015 to 2017), PAR Pharma (sells prepared bags of vasopressin); and he reports having received an investigator-initiated grant from Grifols (entitled “Is HBP a mechanism of albumin’s efficacy in human septic shock?”) that was provided to and administered by UBC. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
Sex, Renin Angiotensin System Inhibitors, and COVID-19 Severity: Biologic Divergence or Healthcare Disparity?Crit Care Med. 2022 Sep 1;50(9):1396-1398. doi: 10.1097/CCM.0000000000005613. Epub 2022 Aug 15. Crit Care Med. 2022. PMID: 35984053 Free PMC article. No abstract available.
-
Can Renin-Angiotensin System Inhibitors Protect Against Acute Kidney Injury in Patients With COVID-19?Crit Care Med. 2022 Nov 1;50(11):e796-e797. doi: 10.1097/CCM.0000000000005618. Epub 2022 Oct 13. Crit Care Med. 2022. PMID: 36227048 Free PMC article. No abstract available.
References
-
- Bader M, Ganten D: Update on tissue renin-angiotensin systems. J Mol Med (Berl) 2008; 86:615–621 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

