The Third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 Delta and Omicron variants
- PMID: 35608053
- PMCID: PMC9176682
- DOI: 10.1080/22221751.2022.2081614
The Third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 Delta and Omicron variants
Abstract
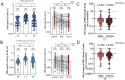
The waning humoral immunity and emerging contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants resulted in the necessity of the booster vaccination of coronavirus disease 2019 (COVID-19). The inactivated vaccine, CoronaVac, is the most widely supplied COVID-19 vaccine globally. Whether the CoronaVac booster elicited adaptive responses that cross-recognize SARS-CoV-2 variants of concern (VoCs) among 77 healthy subjects receiving the third dose of CoronaVac were explored. After the boost, remarkable elevated spike-specific IgG and IgA responses, as well as boosted neutralization activities, were observed, despite 3.0-fold and 5.9-fold reduced neutralization activities against Delta and Omicron strains compared to that of the ancestral strain. Furthermore, the booster dose induced potent B cells and memory B cells that cross-bound receptor-binding domain (RBD) proteins derived from VoCs, while Delta and Omicron RBD-specific memory B cell recognitions were reduced by 2.7-fold and 4.2-fold compared to that of ancestral strain, respectively. Consistently, spike-specific circulating follicular helper T cells (cTfh) significantly increased and remained stable after the boost, with a predominant expansion towards cTfh17 subpopulations. Moreover, SARS-CoV-2-specific CD4+ and CD8+ T cells peaked and sustained after the booster. Notably, CD4+ and CD8+ T cell recognition of VoC spike was largely preserved compared to the ancestral strain. Individuals without generating Delta or Omicron neutralization activities had comparable levels of CD4+ and CD8+ T cells responses as those with detectable neutralizing activities. Our study demonstrated that the CoronaVac booster induced broad and potent adaptive immune responses that could be effective in controlling SARS-CoV-2 Delta and Omicron variants.
Keywords: COVID-19 vaccine; T cell responses; booster; coronavac; neutralization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Amit S, Gonen T, Regev-Yochay G.. Covid-19 breakthrough infections in vaccinated health care workers. Reply. N Engl J Med. 2021 Oct 21;385(17):1630–1631. - PubMed
-
- Centers for Disease Control and Prevention. CDC COVID Data Tracker. (2022). Available online at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
