Diaphragm muscle function in a mouse model of early-onset spasticity
- PMID: 35608200
- PMCID: PMC9255706
- DOI: 10.1152/japplphysiol.00157.2022
Diaphragm muscle function in a mouse model of early-onset spasticity
Abstract
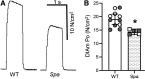
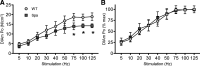
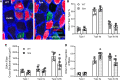
Spasticity is a common symptom in many developmental motor disorders, including spastic cerebral palsy (sCP). In sCP, respiratory dysfunction is a major contributor to morbidity and mortality, yet it is unknown how spasticity influences respiratory physiology or diaphragm muscle (DIAm) function. To investigate the influence of spasticity on DIAm function, we assessed in vivo transdiaphragmatic pressure (Pdi - measured using intraesophageal and intragastric pressure catheters under conditions of eupnea, hypoxia/hypercapnia and occlusion) including maximum Pdi (Pdimax via bilateral phrenic nerve stimulation), ex vivo DIAm-specific force and fatigue (using muscle strips stimulated with platinum plate electrodes), and type-specific characteristics of DIAm fiber cross sections (using immunoreactivity against myosin heavy chain slow and 2A) in spa and wildtype mice. Spa mice show reduced Pdimax, reduced DIAm specific force, and altered fatigability and atrophy of type IIx/IIb fibers. These findings suggest marked DIAm dysfunction may underlie the respiratory phenotype of sCP.NEW & NOTEWORTHY Developmental motor control dysfunctions, including spastic cerebral palsy (sCP) often have respiratory components. Spa mutant mice exhibit a spastic phenotype closely resembling sCP symptoms. Using the spa mouse model of spastic cerebral palsy (sCP), we quantified transdiaphragmatic pressure deficits, diaphragm muscle weakness, and fiber type-specific atrophy, improving our understanding of respiratory dysfunctions in sCP.
Keywords: fatigue; muscle fiber type; muscle-specific force; spasticity; transdiaphragmatic pressure.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109: 8–14, 2007. [Erratum in Dev Med Child Neurol 49: 480, 2007]. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

