The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps
- PMID: 35608258
- PMCID: PMC9129876
- DOI: 10.7554/eLife.72103
The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps
Abstract
Background: Nucleic acid binding proteins are frequently targeted as autoantigens in systemic lupus erythematosus (SLE) and other interferon (IFN)-linked rheumatic diseases. The AIM-like receptors (ALRs) are IFN-inducible innate sensors that form supramolecular assemblies along double-stranded (ds)DNA of various origins. Here, we investigate the ALR absent in melanoma 2 (AIM2) as a novel autoantigen in SLE, with similar properties to the established ALR autoantigen interferon-inducible protein 16 (IFI16). We examined neutrophil extracellular traps (NETs) as DNA scaffolds on which these antigens might interact in a pro-immune context.
Methods: AIM2 autoantibodies were measured by immunoprecipitation in SLE and control subjects. Neutrophil extracellular traps were induced in control neutrophils and combined with purified ALR proteins in immunofluorescence and DNase protection assays. SLE renal tissues were examined for ALR-containing NETs by confocal microscopy.
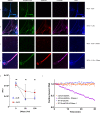
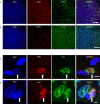
Results: AIM2 autoantibodies were detected in 41/131 (31.3%) SLE patients and 2/49 (4.1%) controls. Our SLE cohort revealed a frequent co-occurrence of anti-AIM2, anti-IFI16, and anti-DNA antibodies, and higher clinical measures of disease activity in patients positive for antibodies against these ALRs. We found that both ALRs bind NETs in vitro and in SLE renal tissues. We demonstrate that ALR binding causes NETs to resist degradation by DNase I, suggesting a mechanism whereby extracellular ALR-NET interactions may promote sustained IFN signaling.
Conclusions: Our work suggests that extracellular ALRs bind NETs, leading to DNase resistant nucleoprotein fibers that are targeted as autoantigens in SLE.
Funding: These studies were funded by NIH R01 DE12354 (AR), P30 AR070254, R01 GM 129342 (JS), K23AR075898 (CM), K08AR077100 (BA), the Jerome L. Greene Foundation and the Rheumatology Research Foundation. Dr. Antiochos and Dr. Mecoli are Jerome L. Greene Scholars. The Hopkins Lupus Cohort is supported by NIH grant R01 AR069572. Confocal imaging performed at the Johns Hopkins Microscopy Facility was supported by NIH Grant S10 OD016374.
Keywords: autoantibodies; autoimmunity; human; immunology; inflammation; medicine; neutrophil extracellular traps.
Plain language summary
Systemic lupus erythematosus (SLE or lupus for short) is an autoimmune disease in which the immune system attacks healthy tissue in organs across the body. The cause is unknown, but people with the illness make antibodies that stick to proteins that are normally found inside the cell nucleus, where DNA is stored. To make these antibodies, the immune system must first ‘see’ these proteins and mistakenly recognise them as a threat. But how does the immune system recognise proteins that are normally hidden inside cells? During infection, a type of immune cell called a neutrophil releases DNA from its nucleus to form structures called neutrophil extracellular traps, or NETs for short. The role of these NETs is to capture and kill pathogens, but they also expose the neutrophil’s DNA and the proteins attached to it to other immune cells. It is therefore possible that other immune cells interacting with NETs during infection may contribute to the development of lupus. Two proteins of interest are AIM2 and IFI16. These proteins form large, shield-like structures around strands of DNA, and previous work has shown that some people with lupus make antibodies against IFI16. Antiochos et al. wondered whether IFI16 and AIM2 might stick to NETs, exposing themselves to the immune system. Examining the blood of people with lupus revealed that one in three of them made antibodies that could stick to AIM2. Those people were also more likely to have antibodies that could stick to IFI16 and to strands of DNA. Using microscopy, Antiochos et al. also found AIM2 and IFI16 on NETs in the kidneys of some people with lupus. Further investigation showed that the presence of AIM2 and IFI16 prevents NETs from breaking down. If proteins like AIM2 and IFI16 can stop NETs from breaking down, they could allow the immune system more time to develop antibodies against them. Further investigation could reveal whether this is one of the causes of lupus. A clearer understanding of the antibodies could also boost research into diagnosis and treatment.
© 2022, Antiochos et al.
Conflict of interest statement
BA, DT, PF, AR, AB, AG, JS, JL, MP, DG, CM, LC, AR No competing interests declared
Figures



References
-
- Antiochos B, Paz M, Li J, Goldman DW, Petri M, Darrah E, Cashman K, Sanz I, Burns KH, Ardeljan D, Andrade F, Rosen A. Autoantibodies targeting LINE-1-encoded ORF1p are associated with systemic lupus erythematosus diagnosis but not disease activity. Clinical and Experimental Rheumatology. 2021;1:bfz387. doi: 10.55563/clinexprheumatol/bfz387. - DOI - PMC - PubMed
-
- Bawadekar M, De Andrea M, Lo Cigno I, Baldanzi G, Caneparo V, Graziani A, Landolfo S, Gariglio M. The Extracellular IFI16 Protein Propagates Inflammation in Endothelial Cells Via p38 MAPK and NF-κB p65 Activation. Journal of Interferon & Cytokine Research. 2015;35:441–453. doi: 10.1089/jir.2014.0168. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

