Gene and protein expression of dorsal root ganglion sensory receptors in normotensive and hypertensive male rats
- PMID: 35608265
- PMCID: PMC9291411
- DOI: 10.1152/ajpregu.00007.2022
Gene and protein expression of dorsal root ganglion sensory receptors in normotensive and hypertensive male rats
Abstract
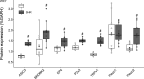
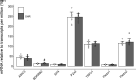
The exercise pressor reflex (EPR), a neurocirculatory control mechanism, is exaggerated in hypertensive humans and rats. Disease-related abnormalities within the afferent arm of the reflex loop, including mechano- and metabosensitive receptors located at the terminal end of group III/IV muscle afferents, may contribute to the dysfunctional EPR in hypertension. Using control (WKY) and spontaneous hypertensive (SHR) rats, we examined dorsal root ganglion (DRG) gene and protein expression of molecular receptors recognized as significant determinants of the EPR. Twelve lumbar DRGs (6 left, 6 right) were harvested from each of 10 WKY [arterial blood pressure (MAP): 96 ± 9 mmHg] and 10 SHR (MAP: 144 ± 9 mmHg). DRGs from the left side were used for protein expression (Western blotting; normalized to GAPDH), whereas right-side DRGs (i.e., parallel structure) were used to determine mRNA levels (RNA-sequencing, normalized to TPM). Analyses focused on metabosensitive (ASIC3, Bradykinin receptor B2, EP4, P2X3, TRPv1) and mechanosensitive (Piezo1/2) receptors. Although Piezo1 was similar in both groups (P = 0.75), protein expression for all other receptors was significantly higher in SHR compared with WKY. With the exception of a greater Bradykinin-receptor B2 in SHR (P < 0.05), mRNA expression of all other receptors was not different between groups (P > 0.18). The higher protein content of these sensory receptors in SHR indirectly supports the previously proposed hypothesis that the exaggerated EPR in hypertension is, in part, due to disease-related abnormalities within the afferent arm of the reflex loop. The upregulated receptor content, combined with normal mRNA levels, insinuates that posttranscriptional regulation of sensory receptor protein expression might be impaired in hypertension.
Keywords: blood pressure; dorsal root ganglion; exercise pressor reflex; hypertension; mRNA.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats.J Physiol. 2011 Dec 15;589(Pt 24):6191-204. doi: 10.1113/jphysiol.2011.214429. Epub 2011 Oct 24. J Physiol. 2011. PMID: 22025666 Free PMC article.
-
Mineralocorticoid receptor antagonists attenuate exaggerated exercise pressor reflex responses in hypertensive rats.Am J Physiol Heart Circ Physiol. 2017 Oct 1;313(4):H788-H794. doi: 10.1152/ajpheart.00155.2017. Epub 2017 Jul 21. Am J Physiol Heart Circ Physiol. 2017. PMID: 28733447 Free PMC article.
-
Voltage-gated potassium channel dysfunction in dorsal root ganglia contributes to the exaggerated exercise pressor reflex in rats with chronic heart failure.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H461-H474. doi: 10.1152/ajpheart.00256.2021. Epub 2021 Jul 16. Am J Physiol Heart Circ Physiol. 2021. PMID: 34270374 Free PMC article.
-
Expression and localization of NK(1)R, substance P and CGRP are altered in dorsal root ganglia neurons of spontaneously hypertensive rats (SHR).Brain Res Mol Brain Res. 2005 Jul 29;138(1):35-44. doi: 10.1016/j.molbrainres.2005.03.015. Brain Res Mol Brain Res. 2005. PMID: 15869822
-
Dynamic exercise training prevents exercise pressor reflex overactivity in spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol. 2015 Sep;309(5):H762-70. doi: 10.1152/ajpheart.00358.2015. Epub 2015 Jul 10. Am J Physiol Heart Circ Physiol. 2015. PMID: 26163445 Free PMC article.
Cited by
-
Hypertension restricts leg blood flow and aggravates neuromuscular fatigue during human locomotion in males.Am J Physiol Regul Integr Comp Physiol. 2024 Nov 1;327(5):R517-R524. doi: 10.1152/ajpregu.00117.2024. Epub 2024 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 39133778
-
The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension?Int J Mol Sci. 2023 May 15;24(10):8769. doi: 10.3390/ijms24108769. Int J Mol Sci. 2023. PMID: 37240118 Free PMC article. Review.
-
Mechanisms of Action of the Peptide Toxins Targeting Human and Rodent Acid-Sensing Ion Channels and Relevance to Their In Vivo Analgesic Effects.Toxins (Basel). 2022 Oct 17;14(10):709. doi: 10.3390/toxins14100709. Toxins (Basel). 2022. PMID: 36287977 Free PMC article. Review.
References
-
- Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi:10.1152/ajpheart.00648.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

