Total Synthesis of (-)-Bastimolide A: A Showcase for Type I Anion Relay Chemistry
- PMID: 35608327
- PMCID: PMC9256807
- DOI: 10.1002/anie.202204884
Total Synthesis of (-)-Bastimolide A: A Showcase for Type I Anion Relay Chemistry
Abstract
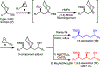
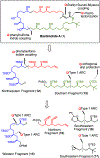
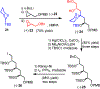
A highly convergent total synthesis of (-)-bastimolide A (1), a polyhydroxy antimalarial macrolide, has been achieved via a longest linear sequence of twenty steps from commercially available glycidyl ethers. Type I Anion Relay Chemistry (ARC) coupling tactics enable rapid construction of the molecule's 1,5-polylol backbone. A late-stage B-alkyl Suzuki-Miyaura union and an Evans-modified Mukaiyama macrolactonization generate the forty-membered Z-α,β-unsaturated macrocyclic lactone.
Keywords: Antimalarial Agents; Macrolides; Multicomponent Coupling; Natural Products; Total Synthesis.
© 2022 Wiley-VCH GmbH.
Figures

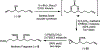






References
-
- Shao C, Linington RG, Balunas MJ, Centeno A, Boudreau P, Zhang C, Engene N, Spadafora C, Mutka TS, Kyle DE, Gerwick L, Wang C, Gerwick WH, J. Org. Chem 2015, 80, 7849–7855; - PubMed
- Salvador LA, Sneed J, Paul VJ, Luesch H, J. Nat. Prod 2015, 78, 1957–1962; - PMC - PubMed
- Salvador LA, Paul VJ, Luesch H, J. Nat. Prod 2010, 73, 1606–1609; - PMC - PubMed
- Mori S, Williams H, Cagle D, Karanovich K, Horgen D, Smith R III, Watanabe CMH, Mar. Drugs 2015, 13, 6274–6290; - PMC - PubMed
- Keller L, Siqueira JL, Souza JM, Eribez K, LaMonte GM, Smith JE, Gerwick WH, Molecules 2020, 25, 1604–1613; - PMC - PubMed
- Shao C, Mou X, Cao F, Spadafora C, Glukhov E, Gerwick L, Wang C, Gerwick WH, J. Nat. Prod 2018, 81, 211–215. - PubMed
-
-
The original structure of caylobolide B reported in ref.1c was incorrect. The geometry of the C2−C3 double bond was revised later by the same group:
- Salvador LA, Paul VJ, Luesch H, J. Nat. Prod 2016, 79, 452. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

