Understanding "Hybrid Immunity": Comparison and Predictors of Humoral Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Infection (SARS-CoV-2) and Coronavirus Disease 2019 (COVID-19) Vaccines
- PMID: 35608504
- PMCID: PMC9213853
- DOI: 10.1093/cid/ciac392
Understanding "Hybrid Immunity": Comparison and Predictors of Humoral Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Infection (SARS-CoV-2) and Coronavirus Disease 2019 (COVID-19) Vaccines
Abstract
Background: Comparison of humoral responses in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinees, those with SARS-CoV-2 infection, or combinations of vaccine/ infection ("hybrid immunity") may clarify predictors of vaccine immunogenicity.
Methods: We studied 2660 US Military Health System beneficiaries with a history of SARS-CoV-2 infection-alone (n = 705), vaccination-alone (n = 932), vaccine-after-infection (n = 869), and vaccine-breakthrough-infection (n = 154). Peak anti-spike-immunoglobulin G (IgG) responses through 183 days were compared, with adjustment for vaccine product, demography, and comorbidities. We excluded those with evidence of clinical or subclinical SARS-CoV-2 reinfection from all groups.
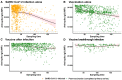
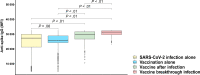
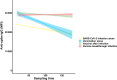
Results: Multivariable regression results indicated that vaccine-after-infection anti-spike-IgG responses were higher than infection-alone (P < .01), regardless of prior infection severity. An increased time between infection and vaccination was associated with greater post-vaccination IgG response (P < .01). Vaccination-alone elicited a greater IgG response but more rapid waning of IgG (P < .01) compared with infection-alone (P < .01). BNT162b2 and mRNA-1273 vaccine-receipt was associated with greater IgG responses compared with JNJ-78436735 vaccine-receipt (P < .01), regardless of infection history. Those with vaccine-after-infection or vaccine-breakthrough-infection had a more durable anti-spike-IgG response compared to infection-alone (P < .01).
Conclusions: Vaccine-receipt elicited higher anti-spike-IgG responses than infection-alone, although IgG levels waned faster in those vaccinated (compared to infection-alone). Vaccine-after-infection elicits a greater humoral response compared with vaccine or infection alone; and the timing, but not disease severity, of prior infection predicted these post-vaccination IgG responses. While differences between groups were small in magnitude, these results offer insights into vaccine immunogenicity variations that may help inform vaccination timing strategies.
Keywords: IgG; SARS-CoV-2; antibody response; vaccine; vaccine breakthrough.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest. S. D. P., T. H. B., D. T., and M. P. S. report that the Uniformed Services University (USU) IDCRP, a US Department of Defense institution, and the Henry M. Jackson Foundation were funded under a cooperative research and development agreement to conduct an unrelated phase 3 COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The Henry M. Jackson Foundation, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase 3 vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US government COVID-19 response. Neither is related to the work presented here. C. M. B. reports a leadership or fiduciary role on the Infectious Diseases Society of America Clinical Affairs Committee. R. C. M. reports grants or contracts to his institution and unrelated to this work from AiCuris, Sound Pharmaceutical, and AstraZeneca; consulting fees and honorarium for advisory panel membership from the Society of Critical Care Medicine; honorarium for a lecture from the California Thoracic Society; travel support from the American Thoracic Society, American College of Chest Physicians, and Society of Critical Care Medicine; a US patent for investigational dengue vaccine candidate (no payments made or current commercial development planned); data and safety monitoring board membership (funds to author) for Trauma Insights, LLC; member of The Society of Critical Care Medicine (SCCM) Congress Program Committee (travel support for official meetings [pre-March 2020]), chair of the American College of Chest Physicians (CHEST) COVID-19 Task Force and Disaster/Global Health Section (travel support for official meetings), and member of the CHEST Scientific Program Committee (travel support for official meetings). All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

