Medical methods for first trimester abortion
- PMID: 35608608
- PMCID: PMC9128719
- DOI: 10.1002/14651858.CD002855.pub5
Medical methods for first trimester abortion
Abstract
Background: Medical abortion became an alternative method of pregnancy termination following the development of prostaglandins and antiprogesterone in the 1970s and 1980s. Recently, synthesis inhibitors of oestrogen (such as letrozole) have also been used to enhance efficacy. The most widely researched drugs are prostaglandins (such as misoprostol, which has a strong uterotonic effect), mifepristone, mifepristone with prostaglandins, and letrozole with prostaglandins. More evidence is needed to identify the best dosage, regimen, and route of administration to optimise patient outcomes. This is an update of a review last published in 2011.
Objectives: To compare the effectiveness and side effects of different medical methods for first trimester abortion.
Search methods: We searched CENTRAL, MEDLINE, Embase, Global Health, and LILACs on 28 February 2021. We also searched Clinicaltrials.gov and the World Health Organization's (WHO) International Clinical Trials Registry Platform, and reference lists of retrieved papers.
Selection criteria: We considered randomised controlled trials (RCTs) that compared different medical methods for abortion before the 12th week of gestation. The primary outcome is failure to achieve complete abortion. Secondary outcomes are mortality, surgical evacuation, ongoing pregnancy at follow-up, time until passing of conceptus, blood transfusion, side effects and women's dissatisfaction with the method.
Data collection and analysis: Two review authors independently selected and evaluated studies for inclusion, and assessed the risk of bias. We processed data using Review Manager 5 software. We assessed the certainty of the evidence using the GRADE approach.
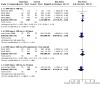
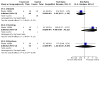
Main results: We included 99 studies in the review (58 from the original review and 41 new studies). 1. Combined regimen mifepristone/prostaglandin Mifepristone dose: high-dose (600 mg) compared to low-dose (200 mg) mifepristone probably has similar effectiveness in achieving complete abortion (RR 1.07, 95% CI 0.87 to 1.33; I2 = 0%; 4 RCTs, 3494 women; moderate-certainty evidence). Prostaglandin dose: 800 µg misoprostol probably reduces abortion failure compared to 400 µg (RR 0.63, 95% CI 0.51 to 0.78; I2= 0%; 3 RCTs, 4424 women; moderate-certainty evidence). Prostaglandin timing: misoprostol administered on day one probably achieves more success on complete abortion than on day three (RR 1.94, 95% CI 1.05 to 3.58; 1489 women; 1 RCT; moderate-certainty evidence). Administration strategy: there may be no difference in failure of complete abortion with self-administration at home compared with hospital administration (RR 1.63, 95% CI 0.68 to 3.94; I2 = 84%; 2263 women; 4 RCTs; low-certainty evidence), but failure may be higher when administered by nurses in hospital compared to by doctors in hospital (RR 2.69, 95% CI 1.39 to 5.22; I2 = 66%; 3 RCTs, 3056 women; low-certainty evidence). Administration route: oral misoprostol probably leads to more failures than the vaginal route (RR 2.38, 95% CI 1.46 to 3.87; I2 = 39%; 3 RCTs, 1704 women; moderate-certainty evidence) and may be associated with more frequent side effects such as nausea (RR 1.14, 95% CI 1.03 to 1.26; I2 = 0%; 2 RCTs, 1380 women; low-certainty evidence) and diarrhoea (RR 1.80 95% CI 1.49 to 2.17; I2 = 0%; 2 RCTs, 1379 women). Compared with the vaginal route, complete abortion failure is probably lower with sublingual (RR 0.68, 95% CI 0.22 to 2.11; I2 = 59%; 2 RCTs, 3229 women; moderate-certainty evidence) and may be lower with buccal administration (RR 0.71, 95% CI 0.34 to 1.46; I2 = 0%; 2 RCTs, 479 women; low-certainty evidence), but sublingual or buccal routes may lead to more side effects. Women may experience more vomiting with sublingual compared to buccal administration (RR 1.33, 95% CI 1.01 to 1.77; low-certainty evidence). 2. Mifepristone alone versus combined regimen The efficacy of mifepristone alone in achieving complete abortion compared to combined mifepristone/prostaglandin up to 12 weeks is unclear (RR of failure 3.25, 95% CI 0.81 to 13.09; I2 = 83%; 3 RCTs, 273 women; very low-certainty evidence). 3. Prostaglandin alone versus combined regimen Nineteen studies compared prostaglandin alone to a combined regimen (prostaglandin combined with mifepristone, letrozole, estradiol valerate, tamoxifen, or methotrexate). Compared to any of the combination regimens, misoprostol alone may increase the risk for failure to achieve complete abortion (RR of failure 2.39, 95% CI 1.89 to 3.02; I2 = 64%; 18 RCTs, 3471 women; low-certainty evidence), and with more diarrhoea. 4. Prostaglandin alone (route of administration) Oral misoprostol alone may lead to more failures in complete abortion than the vaginal route (RR 3.68, 95% CI 1.56 to 8.71, 2 RCTs, 216 women; low-certainty evidence). Failure to achieve complete abortion may be slightly reduced with sublingual compared with vaginal (RR 0.69, 95% CI 0.37 to 1.28; I2 = 87%; 5 RCTs, 2705 women; low-certainty evidence) and oral administration (RR 0.58, 95% CI 0.11 to 2.99; I2 = 66%; 2 RCTs, 173 women). Failure to achieve complete abortion may be similar or slightly higher with sublingual administration compared to buccal administration (RR 1.11, 95% CI 0.71 to 1.74; 1 study, 401 women).
Authors' conclusions: Safe and effective medical abortion methods are available. Combined regimens (prostaglandin combined with mifepristone, letrozole, estradiol valerate, tamoxifen, or methotrexate) may be more effective than single agents (prostaglandin alone or mifepristone alone). In the combined regimen, the dose of mifepristone can probably be lowered to 200 mg without significantly decreasing effectiveness. Vaginal misoprostol is probably more effective than oral administration, and may have fewer side effects than sublingual or buccal. Some results are limited by the small numbers of participants on which they are based. Almost all studies were conducted in settings with good access to emergency services, which may limit the generalisability of these results.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JZ: nothing to declare
KZ: nothing to declare
DS: nothing to declare
XL: nothing to declare
Figures









































































































Update of
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2011 Nov 9;2011(11):CD002855. doi: 10.1002/14651858.CD002855.pub4. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2022 May 24;5:CD002855. doi: 10.1002/14651858.CD002855.pub5. PMID: 22071804 Free PMC article. Updated.
Comment in
-
Medical Methods for First-Trimester Abortion.Am Fam Physician. 2023 Jan;107(1):24-25. Am Fam Physician. 2023. PMID: 36689964 No abstract available.
References
References to studies included in this review
Abbasalizadeh 2018 {published data only}
-
- Abbasalizadeh F, Sahhaf F, Sadeghi-Shabestari P, Mirza-Aghazadeh-Attari M, Naghavi-Behzad M.Comparison between effect of letrozole plus misoprostol and misoprostol alone in terminating non-viable first trimester pregnancies: a single blind randomized trial. Journal of Family and Reproductive Health 2018;12(1):27-33. - PMC - PubMed
Abdelshafy 2019 {published data only}
-
- Abdelshafy A, Awwad H, Abo-Gamra A, Alanwar A, Elkotb AM, Shahin M, et al.Sublingual vs vaginal misoprostol for completion of first trimester missed abortion: a randomised controlled trial. European Journal of Contraception & Reproductive Health Care 2019;24(2):134-9. [DOI: ] - PubMed
Allameh 2020 {published data only}
-
- Allameh Z, Goharian M, Eslamian M.Share effect of misoprostol with and without letrozole on the induction of abortion for women with first-trimester missed abortion. International Journal of Gynaecology and Obstetrics 2020;151(2):214-8. - PubMed
Arvidsson 2005 {published data only}
-
- Arvidsson C, Hellborg M, Gemzell-Daniellson K.Preference and acceptability of oral versus vaginal administration of misoprostol in medical abortion with mifepristone. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123:87-91. - PubMed
Baird 1995 {published data only}
-
- Baird DT, Sukcharoen N, Thong KJ.Randomized trial of misoprostol and cervagem in combination with a reduced dose of mifepristone for induction of abortion. Human Reproduction (Oxford, England) 1995;10(6):1521-7. - PubMed
Bartley 2001 {published data only}
-
- Bartley J, Brown A, Elton R, Baird DT.Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation. Human Reproduction (Oxford, England) 2001;16(10):2098-102. - PubMed
Behroozi Lak 2018 {published data only}
-
- Behroozi-Lak T, Derakhshan-Aydenloo S, Broomand F.Evaluation of effect of letrozole prior to misoprostol in comparison with misoprostol alone in success rate of induced abortion. Journal of Gynecology Obstetrics and Human Reproduction 2018;47(3):113-7. [DOI: ] - PubMed
Birgerson 1988 {published data only}
-
- Birgerson L, Odlind V.The antiprogestational agent RU 486 as an abortifacient in early human pregnancy: a comparison of three dose regimens. Contraception 1988;38(4):391-400. - PubMed
Blanchard 2005 {published data only}
-
- Blanchard K, Tara Shochet T, Coyajic K, Ngoc N, Winikoff B.Misoprostol alone for early abortion: an evaluation of seven potential regimens. Contraception 2005;72:91-7. - PubMed
Blum 2012 {published data only}
-
- Blum J, Raghavan S, Dabash R, Ngoc NtN, Chelli H, Hajri S, et al.Comparison of misoprostol-only and combined mifepristone-misoprostol regimens for home-based early medical abortion in Tunisia and Vietnam. International Journal of Gynaecology and Obstetrics 2012;118(2):166-71. [DOI: ] - PubMed
Cameron 1986 {published data only}
-
- Cameron IT, Michie AF, Baird DT.Therapeutic abortion in early pregnancy with antiprogestogen RU 486 alone or in combination with prostaglandin analogue (gemeprost). Contraception 1986;34(5):459-68. - PubMed
Carbonell 1997b {published data only}
-
- Carbonell JL, Velazco A, Varela L, Cabezas E, Fernandez C, Sanchez C.Misoprostol 3, 4, or 5 days after methotrexate for early abortion. Contraception 1997;56:169-74. - PubMed
Carbonell 1998 {published data only}
-
- Carbonell JL, Varela L, Velazco A, Cabezas E, Fernandez C, Sanchez C.Oral methotrexate and vaginal misoprostol for early abortion. Contraception 1998;57:83-8. - PubMed
Chai 2013 {published data only}
-
- Chai J, Wong CY, Ho PC.A randomized clinical trial comparing the short-term side effects of sublingual and buccal routes of misoprostol administration for medical abortions up to 63 days' gestation. Contraception 2013;87(4):480-5. [DOI: ] - PubMed
Chen 2015a {published data only}
-
- Chen LY, Hu LS, Chen XY, Ming ZS.Clinical study on the synergistic effect of medical abortion of missed abortion with Shenghua decoction Jiawei prescription. Journal of Jiangxi Univeristy of TCM 2015;27(3):39.
Cheng 1994 {published data only}
-
- Cheng LN, Zhou YF, Song JY, Li H, Chen JK.A randomized clinical trial in comparison of termination of early pregnancy by 16, 16-dimethyl-trans PGE1 ester alone or in combination with testosterone propionate. Sheng zhi yu bi yun (Reproduction and Contraception) 1994;1:29-33.
Chong 2012 {published data only}
-
- Chong E, Tsereteli T, Nguyen NN, Winikoff B.A randomized controlled trial of different buccal misoprostol doses in mifepristone medical abortion. Contraception 2012;86(3):251-6. [DOI: ] - PubMed
Coyaji 2007 {published data only}
-
- Coyaji K, Krishna U, Ambardekar S, Bracken H, Raote V, Mandlekar A, et al.Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG 2007;114:271–8. - PubMed
Creinin 1994 {published data only}
-
- Creinin DM, Vittinghoff E.Methotrexate and misoprostol vs misoprostol alone for early abortion. JAMA 1994;272(15):1190-5. - PubMed
Creinin 1995 {published data only}
-
- Creinin MD, Vittinghoff E, Galbraith S, Klaisle C.A randomized trial comparing misoprostol three and seven days after methotrexate for early abortion. American Journal of Obstetrics and Gynecology 1995;173:1578-84. - PubMed
Creinin 1996a {published data only}
-
- Creinin MD.Oral methotrexate and vaginal misoprostol for early abortion. Contraception 1996;54:15-8. - PubMed
Creinin 1997 {published data only}
-
- Creinin MD, Krohn MA.Methotrexate pharmacokinetics and effects in women receiving methotrexate 50 mg and 60 mg per square meter for early abortion. American Journal of Obstetrics and Gynecology 1997;177:1444-9. - PubMed
Creinin 2001a {published data only}
-
- Creinin MD, Pymar HC, Schwartz JL.Mifepristone 100 mg in abortion regimens. Obstetrics and Gynecology 2001;98(3):434-9. - PubMed
Creinin 2001b {published data only}
-
- Creinin MD, Schwartz JL, Pymar HC, Fink W.Efficacy of mifepristone followed on the same day by misoprostol for early termination of pregnancy: report of a randomised trial. British Journal of Obstetrics and Gynaecology 2001;108:469-73. - PubMed
Creinin 2004 {published data only}
-
- Creinin MD, Fox MC, Teal S, Chen A, Schaff EA, Meyn LA.A randomized comparison of misoprostol 6 to 8 hours versus 24 hours after mifepristone for abortion. Obstetrics and Gynecology 2004;103:851-9. - PubMed
Creinin 2007 {published data only}
-
- Creinin MD, Schreiber CA, Bednarek P, Lintu H, Wagner MS, Meyn LA.Mifepristone and misoprostol administered simultaneously versus 24 hours apart for abortion. Obstetrics and Gynecology 2007;109:885-94. - PubMed
Dahiya 2011 {published data only}
-
- Dahiya K, Mann S, Nanda S.Randomized trial of oral versus sublingual misoprostol 24 h after mifepristone for medical abortion. Archives of Gynecology and Obstetrics 2011;284(1):59-63. [DOI: ] - PubMed
El‐Refaey 1994 {published data only}
-
- El-Refaey H, Templeton A.Early abortion induction by a combination of mifepristone and oral misoprostol: a comparison between two dose regimens of misoprostol and their effect on blood pressure. British Journal of Obstetrics and Gynaecology 1994;101:792-6. - PubMed
El‐Refaey 1995 {published data only}
-
- El-Refaey H, Rajasekar D, Abdalla M, Calder L, Templeton A.Induction of abortion with mifepristone (RU 486) and oral or vaginal misoprostol. New England Journal of Medicine 1995;332:983-7. - PubMed
Fekih 2010 {published data only}
-
- Fekih M, Fathallah K, Ben Regaya L, Bouguizane S, Chaieb A, Bibi M, et al.Sublingual misoprostol for first trimester termination of pregnancy. International Journal of Gynaecology and Obstetrics 2010;109(1):67-70. [DOI: ] - PubMed
Garg 2015 {published data only}
Goel 2011 {published data only}
-
- Goel A, Mittal S, Taneja BK, Singal N, Attri S.Simultaneous administration of mifepristone and misoprostol for early termination of pregnancy: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(6):1409-13. [DOI: ] - PubMed
Guest 2007 {published data only}
-
- Guest J, Chien PF, Thomson MA, Kosseim ML.Randomised controlled trial comparing the efficacy of same-day administration of mifepristone and misoprostol for termination of pregnancy with the standard 36 to 48 hour protocol. BJOG 2007;114:207-15. - PubMed
Hamoda 2005 {published data only}
-
- Hamoda H, Ashok PW, Flett GM, Templeton A.A randomised controlled trial of mifepristone in combination with misoprostol administered sublingually or vaginally for medical abortion up to 13 weeks of gestation. BJOG 2005;112:1102–8. - PubMed
Iyengar 2015 {published data only}
-
- Iyengar K, Klingberg-Allvin M, Iyengar SD, Paul M, Essen B, Gemzell-Danielsson K.Home use of misoprostol for early medical abortion in a low resource setting: secondary analysis of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2016;95(2):173-81. [DOI: ] - PubMed
-
- Iyengar K, Paul M, Iyengar SD, Klingberg-Allvin M, Essen B, Bring J, et al.Self-assessment of the outcome of early medical abortion versus clinic follow-up in India: a randomised, controlled, non-inferiority trial. Lancet 2015;3(9):e537-45. [DOI: ] - PubMed
Jain 1999 {published data only}
-
- Jain JK, Meckstroth KR, Park M, Mishell DR Jr.A comparison of tamoxifen and misoprostol to misoprostol alone for early pregnancy termination. Contraception 1999;60(6):353-6. - PubMed
Jain 2002 {published data only}
-
- Jain JK, Dutton C, Harwood B, Meckstroth KR, Mishell DR.A prospective randomized, double-blinded, placebo-controlled trial comparing mifepristone and vaginal misoprostol to vaginal misoprostol alone for elective termination of early pregnancy. Human Reproduction (Oxford, England) 2002;17(6):1477-82. - PubMed
Javadi 2015 {published data only}
-
- Javadi EH, Mohammadi M, Barikani A.Induction of abortion in the first trimester by misoprostol or misoprostol with letrozole. Biotechnology and Health Sciences 2015;2(3):e29562.
Klingberg Allvin 2015 {published data only}
-
- Cleeve A, Byamugisha J, Gemzell-Danielsson K, Mbona Tumwesigye N, Atuhairwe S, Faxelid E, et al.Women's acceptability of misoprostol treatment for incomplete abortion by midwives and physicians - secondary outcome analysis from a randomized controlled equivalence trial at district level in Uganda. PloS One 2016;11(2):e0149172. [DOI: ] - PMC - PubMed
-
- Klingberg-Allvin M, Cleeve A, Atuhairwe S, Tumwesigye NM, Faxelid E, Byamugisha J, et al.Comparison of treatment of incomplete abortion with misoprostol by physicians and midwives at district level in Uganda: a randomised controlled equivalence trial. Lancet 2015;385(9985):2392-8. [DOI: ] - PubMed
Koopersmith 1996 {published data only}
-
- Koopersmith TB, Mishell DR.The use of misoprostol for termination of early pregnancy. Contraception 1996;53:237-42. - PubMed
Kopp Kallner 2015 {published data only}
-
- Kallner HK, Gomperts R, Salomonsson E, Johansson M, Marions L, Danielsson KG.The efficacy, safety, and acceptability of medical abortion provided by nurse-midwives or physicians-a randomized controlled equivalence trial. European Journal of Contraception and Reproductive Health Care 2014;19:S84-5. [DOI: 10.3109/13625187.2014.894779.10] - DOI
-
- Kopp Kallner H, Gomperts R, Salomonsson E, Johansson M, Marions L, Gemzell-Danielsson K.The efficacy, safety and acceptability of medical termination of pregnancy provided by standard care by doctors or by nurse-midwives: a randomised controlled equivalence trial. BJOG 2015;122(4):510-7. [DOI: ] - PubMed
Lee 2011 {published data only}
-
- Lee VC, Ng EH, Yeung WS, Ho PC.Misoprostol with or without letrozole pretreatment for termination of pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2011;117(2 Pt 1):317-23. [DOI: ] - PubMed
Li 2015 {published data only}
Li 2017 {published data only}
-
- Li CL, Song LP, Tang SY, Zhou LJ, He H, Mo XT, et al.Efficacy, safety, and acceptability of low-dose mifepristone and self-administered misoprostol for ultra-early medical abortion: a randomized controlled trial. Reproductive Sciences 2017;24(5):731-7. [DOI: ] - PubMed
Liao 2004 {published data only}
-
- Liao A, Han XJ, Wu SY, Xiao DZ, Xiong CL, Wu XR.Randomized, double-blind, controlled trial of mifepristone in capsule versus tablet form followed by misoprostol for early medical abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2004;116:211-6. - PubMed
Marwah 2016 {published data only}
McKinley 1993 {published data only}
-
- McKinley C, Thong KJ, Baird DT.The effect of dose of mifepristone and gestation on the efficacy of medical abortion with mifepristone and misoprostol. Human Reproduction (Oxford, England) 1993;8(9):1502-5. - PubMed
Middleton 2005 {published data only}
-
- Middleton T, Schaff E, Fielding SL, Scahill M, Shannon C, Westheimer E, et al.Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period. Contraception 2005;72:328-32. - PubMed
Mizrachi 2017 {published data only}
-
- Mizrachi Y, Dekalo A, Gluck O, Miremberg H, Dafna L, Feldstein O, et al.Single versus repeat doses of misoprostol for treatment of early pregnancy loss-a randomized clinical trial. Human Reproduction (Oxford, England) 2017;32(6):1202-7. [DOI: ] - PubMed
Ng 2015 {published data only}
-
- Ng BK, Annamalai R, Lim PS, Aqmar Suraya S, Nur Azurah AG, Muhammad Abdul Jamil MY.Outpatient versus inpatient intravaginal misoprostol for the treatment of first trimester incomplete miscarriage: a randomised controlled trial. Archives of Gynecology and Obstetrics 2015;291(1):105-13. [DOI: ] - PubMed
Ngoc 2011 {published data only}
-
- Ngoc NT, Blum J, Raghavan S, Nga NT, Dabash R, Diop A, et al.Comparing two early medical abortion regimens: mifepristone+misoprostol vs. misoprostol alone. Contraception 2011;83(5):410-7. [DOI: ] - PubMed
Olavarrieta 2015 {published data only}
Ozeren 1999 {published data only}
-
- Ozeren M, Bilekli C, Aydemir V, Bozkaya H.Methotrexate and misoprostol used alone or in combination for early abortion. Contraception 1999;59:389-94. - PubMed
Paritakul 2010 {published data only}
-
- Paritakul P, Phupong V.Comparative study between oral and sublingual 600 micro g misoprostol for the treatment of incomplete abortion. Journal of Obstetrics and Gynaecology Research 2010;36(5):978-83. [DOI: ] - PubMed
Qian 2015 {published data only}
-
- Qian J, Jing X, Wu S, Zheng S, Li Y, Ren M, et al.Efficacy and safety of mifepristone combined with misoprostol for termination of pregnancy between 8 and 16 weeks of gestation. Zhonghua Fu Chan Ke Za Zhi 2015;50(7):505-9. - PubMed
Raghavan 2009 {published data only}
-
- Raghavan S, Comendant R, Digol I, Ungureanu S, Friptu V, Bracken H, et al.Two-pill regimens of misoprostol after mifepristone medical abortion through 63 days' gestational age: a randomized controlled trial of sublingual and oral misoprostol. Contraception 2009;79:84-90. - PubMed
Raghavan 2010 {published data only}
-
- Raghavan S, Comendant R, Digol I, Ungureanu S, Dondiuc I, Turcanu S, et al.Comparison of 400 mcg buccal and 400 mcg sublingual misoprostol after mifepristone medical abortion through 63 days' LMP: a randomized controlled trial. Contraception 2010;82(6):513-9. [DOI: ] - PubMed
Rodger 1989 {published data only}
-
- Rodger MW, Logan AF, Baird DT.Induction of early abortion with mifepristone (RU 486) and two different doses of prostaglandin pessary (gemeprost). Contraception 1989;39(5):497-502. - PubMed
Sandstrom 1999 {published data only}
-
- Sandstrom O, Brooks L, Schantz A, Grinsted J, Grinsted L, Jacobsen JD, et al.Interruption of early pregnancy with mifepristone in combination with gemeprost. Acta Obstetricia et Gynecologica Scandinavica 1999;78:806-9. - PubMed
Sang 1994 {published data only}
-
- Sang GW, Weng LJ, Shao QX, Du MK, Wu XZ, Lu YL, et al.Termination of early pregnancy by two regimens of mifepristone with misoprostol and mifepristone with PG05 - a multicentre randomized clinical trial in China. Contraception 1994;50:501-10. - PubMed
Sang 1999 {published data only}
-
- Sang GW, He CH, Shao QX, Zhuang LQ, Weng LJ, Wu BX, et al.A large scale introductory trial on termination of early pregnancy by mifepristone in combination with different prostaglandins. Chinese Journal for Clinical Pharmacology 1999;15(5):323-9.
Schaff 2000 {published data only}
-
- Schaff EA, Fielding SL, Westhoff C, Ellertson C, Eisinger SH, Stadalius LS, et al.Vaginal misoprostol administered 1, 2 or 3 days after mifepristone for early medical abortion. JAMA 2000;284(15):1948-53. - PubMed
Schaff 2001 {published data only}
-
- Schaff EA, Fielding SL, Westhoff C.Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception 2001;64(2):81-5. - PubMed
Schreiber 2018 {published data only}
Shannon 2006 {published data only}
-
- Shannon C, Wiebe E, Jacot F, Guilbert E, Dunn S, Sheldon W, et al.Regimens of misoprostol with mifepristone for early medical abortion: a randomised trial. BJOG 2006;113:621–8. - PubMed
Sheldon 2019 {published data only}
-
- Sheldon WR, Durocher J, Dzuba IG, Sayette H, Martin R, Velasco MC, et al.Early abortion with buccal versus sublingual misoprostol alone: a multicenter, randomized trial. Contraception 2019;99(5):272-7. [DOI: ] - PubMed
Shrestha 2014 {published data only}
-
- Shrestha A, Sedhai LB.A randomized trial of hospital vs home self administration of vaginal misoprostol for medical abortion. Kathmandu University Medical Journal 2014;12(47):185-9. - PubMed
Sinha 2018 {published data only}
-
- Sinha P, Suneja A, Guleria K, Aggarwal R, Vaid NB.Comparison of mifepristone followed by misoprostol with misoprostol alone for treatment of early pregnancy failure: a randomized double-blind placebo-controlled trial. Journal of Obstetrics and Gynaecology of India 2018;68(1):39-44. [DOI: ] - PMC - PubMed
Song 2018 {published data only}
Sonsanoh 2014 {published data only}
-
- Sonsanoh A, Chullapram T.Comparison of sublingual and vaginal misoprostol for termination of early pregnancy failure: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2014;22(3):128.
Souizi 2020 {published data only}
-
- Souizi B, Akrami R, Borzoee F, Sahebkar M.Comparison of the efficacy of sublingual, oral, and vaginal administration of misoprostol in the medical treatment of missed abortion during first trimester of pregnancy: a randomized clinical trial study. Journal of Research in Medical Sciences 2020;25:72. - PMC - PubMed
Swahn 1989 {published data only}
-
- Swahn ML, Ugocsai G, Bygdeman M, Kovacs L, Belsey EM, Van Look PF.Effect of oral prostaglandin E2 on uterine contractility and outcome of treatment in women receiving RU 486 (mifepristone) for termination of early pregnancy. Human Reproduction (Oxford, England) 1989;4(1):21-8. - PubMed
Tang 2002 {published data only}
-
- Tang OS, Lee SW, Ho PC.A prospective randomized study on the measured blood loss in medical termination of early pregnancy by three different misoprostol regimens after pretreatment with mifepristone. Human Reproduction (Oxford, England) 2002;17(11):2865-8. - PubMed
Tang 2003 {published data only}
-
- Tang OS, Chan CC, Ng EH, Lee SW and Ho PC.A prospective, randomized, placebo-controlled trial on the use of mifepristone with sublingual or vaginal misoprostol for medical abortions of less than 9 weeks gestation. Human Reproduction (Oxford, England) 2003;18:2315-8. - PubMed
Tanha 2010 {published data only}
Teimoori 2019 {published data only}
-
- Teimoori B, Esmaeilpoor M, Ashkezari AK, Farzaneh F.Comparison of induction abortion in the first trimester using misoprostol alone and misoprostol with estrogen priming. International Journal of Women’s Health and Reproduction Sciences 2019;7(3):404.
Torky 2018 {published data only}
Vahid Roudsari 2010 {published data only}
Verma 2011 {published data only}
-
- Verma ML, Singh U, Singh N, Shankhwar P, Srivastava D.Efficacy of misoprostol administration 24 hours after mifepristone for termination of early pregnancy. Indian Journal of Medical Sciences 2010;65(12):511-7. - PubMed
Verma 2017 {published data only}
-
- Verma ML, Singh U, Singh N, Sankhwar PL, Qureshi S.Efficacy of concurrent administration of mifepristone and misoprostol for termination of pregnancy. Human Fertility 2017;20(1):43-7. - PubMed
von Hertzen 2003 {published data only}
-
- Honkanen H, Piaggio G, Von Hertzen H, Bartfai G, Erdenetungalag R, Gemzell-Danielsson K, et al.WHO multinational study of three misoprostol regimens after mifepristone for early medical abortion II: side effects and women’s perceptions. BJOG 2004;111:715-25. - PubMed
-
- Von Hertzen H, Honkanen H, Piaggio G, Bartfai G, Erdenetungalag R, Gemzell-Danielsson K, et al.WHO multinational study of three misoprostol regimens after mifepristone for early medical abortion. I: efficacy. BJOG 2003;110:808-18. - PubMed
von Hertzen 2007 {published data only}
-
- Hertzen H, Piaggio G, Huong N, Arustamyan K, Cabezas E, Gomez M, et al, on behalf of the WHO Research Group on Post-Ovulatory Methods of Fertility Regulation.Efficacy of two intervals and two routes of administration of misoprostol for termination of early pregnancy: a randomised controlled equivalence trial. Lancet 2007;369:1938-46. - PubMed
von Hertzen 2009 {published data only}
-
- Hertzen H, Piaggio G, Wojdyla D, Marions L, My Huong NT, Tang OS, et al, for the WHO Research Group on Post-Ovulatory Methods of Fertility Regulation.Two mifepristone doses and two intervals of misoprostol administration for termination of early pregnancy: a randomised factorial controlled equivalence trial. BJOG 2009;116:381–9. - PubMed
von Hertzen 2010 {published data only}
-
- Hertzen H, Huong NT, Piaggio G, Bayalag M, Cabezas E, Fang AH, et al, WHO Research Group on Post-Ovulatory Methods of Fertility Regulation.Misoprostol dose and route after mifepristone for early medical abortion: a randomised controlled noninferiority trial. BJOG 2010;117(10):1186-96. [DOI: ] - PubMed
Wang 2000 {published data only}
-
- Wang ZH.Prevent from bleeding after medical abortion through prolonged application of mifepristone with misoprostol for terminating early pregnancy. Zhonghua Fu Chan Ke Za Zhi 2000;35(9):554-7. - PubMed
Warriner 2011 {published data only}
-
- Warriner IK, Wang D, Huong NTM, Thapa K, Tamang A, Shah I, et al.Can midlevel health-care providers administer early medical abortion as safely and effectively as doctors? A randomised controlled equivalence trial in Nepal. Lancet 2011;377(9772):1155-61. [DOI: ] - PubMed
WHO 1989 {published data only}
-
- WHO Task Force on Post-Ovulatory Methods for Fertility Regulation.Termination of early human pregnancy with RU 486 (mifepristone) and the prostaglandin analogue sulprostone: a multi-centre, randomized comparison between two treatment regimens. Human Reproduction (Oxford, England) 1989;4(6):718-25. - PubMed
WHO 1991 {published data only}
-
- WHO Task Force on Post-Ovulatory Methods for Fertility Regulation.Pregnancy termination with mifepristone and gemeprost: a multicenter comparison between repeated doses and a single dose of mifepristone. Fertility and Sterility 1991;56:32-40. - PubMed
WHO 1993 {published data only}
WHO 2000 {published data only}
-
- WHO Task Force on Post-Ovulatory Methods of Fertility Regulation.Comparison of two doses of mifepristone in combination with misoprostol for early medical abortion: a randomised trial. BJOG 2000;107:524-30. - PubMed
WHO 2001a {published data only}
-
- World Health Organization Task Force on Post-Ovulatory Methods of Fertility Regulation.Medical abortion at 57 to 63 days' gestation with a lower dose of mifepristone and gemeprost. Acta Obstetrica et Gynecologica Scandinavica 2001;80:447-51. - PubMed
WHO 2001b {published data only}
-
- WHO Task Force on Post-Ovulatory Methods for Fertility Regulation.Lowering the doses of mifepristone and gemeprost for early abortion: a randomised controlled trial. BJOG 2001;108:738-42. - PubMed
Wiebe 1999a {published data only}
-
- Wiebe ER.Tamoxifen compared to methotrexate when used with misoprostol for abortion. Contraception 1999;59:265-70. - PubMed
Wiebe 1999b {published data only}
-
- Wiebe ER.Oral methotrexate compared with injected methotrexate when used with misoprostol for abortion. American Journal of Obstetrics & Gynecology 1999;181:149-52. - PubMed
Wiebe 2004 {published data only}
-
- Wiebe ER, Trouton K.Comparing vaginal and buccal misoprostol when used after methotrexate for early abortion. Contraception 2004;70:463-6. - PubMed
Wiebe 2006 {published data only}
-
- Wiebe ER, Trouton KJ, Lima R.Misoprostol alone vs. methotrexate followed by misoprostol for early abortion. International Journal of Gynecology and Obstetrics 2006;95:286-7. - PubMed
Winikoff 2008 {published data only}
-
- Winikoff B, Dzuba IG, Creinin MD, Crowden WA, Goldberg AB, Gonzales J, et al.Two distinct oral routes of misoprostol in mifepristone medical abortion: a randomized controlled trial. Obstetrics and Gynecology 2008;112(6):1303-10. - PubMed
Wu 1993 {published data only}
-
- Wu YM, Li Y, Fan HM, Zhu XJ, Zheng S, Wang S, et al.Clinical study of mifepristone in combination with tamoxifen and 15-methyl-PGF2alpha methylester for termination of early pregnancy. Sheng Zhi Yi Xue Za Zhi 1993;4:224-7.
Zheng 1989 {published data only}
-
- Zheng SR.RU486 (mifepristone): clinical trials in China. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1989;149:19-23. - PubMed
References to studies excluded from this review
ACTRN12612001254886 {published data only}
-
- ACTRN12612001254886.Mifipristone and misoprostol compared to misoprostol alone in the treatment of first trimester miscarriage. A randomised control trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363306 (first received 26 November 2012).
ACTRN12613001230741 {published data only}
-
- ACTRN12613001230741.Can nurses offer early medical abortion as safely and effectively as physicians? A randomized controlled non-inferiority trial in Mexico City public legal abortion sites. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365004 (first received 26 September 2013).
Aggarwal 2017 {published data only}
-
- Aggarwal P, Mittal S, Gupta N, Patra L, Singh D.Mifepristone and misoprostol for the termination of pregnancy at 64-140 days from LMP. BJOG 2017;124:54.
Ashok 2002 {published data only}
-
- Ashok PW, Templeton A, Wagaarchchi PT, Flett GM.Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG 2002;109(11):1281-9. - PubMed
Aubeny 2000 {published data only}
-
- Aubeny E, Chatellier G.A randomized comparison of mifepristone and self-administered oral or vaginal misoprostol for early abortion. European Journal of Contraception & Reproductive Health Care 2000;5(3):171-6. - PubMed
Chelly 2010 {published data only}
-
- Chelly D, Najar I, Boudaya F, Affes M, Zouaoui B, Sfar E, et al.Erratum: two medical abortion regimens for late first-trimester termination of pregnancy: a prospective randomized trial. Contraception 2010;82(2):217. - PubMed
Cheng 1999 {published data only}
-
- Cheng L.Termination of 10-16 weeks' gestation with mifepristone plus misoprostol: a multicentre randomized clinical trial. Zhonghua Fu Chan Ke za Zhi 1999;34(5):268-71. - PubMed
ChiCTR1900025763 {published data only}
-
- ChiCTR1900025763.A randomized controlled trial for vaginal versus sublingual misoprostol in early first-trimester medical abortion. chictr.org.cn/showproj.aspx?proj=42993 (registration date 7 September 2019).
ChiCTR‐TRC‐11001438 {published data only}
-
- ChiCTR-TRC-11001438.Combination of mifepristone and misoprostol for termination of 8-16 weeks' pregnancy: a multicenter, randomized controlled study. chictr.org.cn/showproj.aspx?proj=8101 (date of registration 8 July 2011).
Creinin 1996b {published data only}
-
- Creinin MD, Burke AE.Methotrexate and misoprostol for early abortion: a multicenter trial. Acceptability. Contraception 1996;54:19-22. - PubMed
CTRI/2011/07/001894 {published data only}
-
- CTRI/2011/07/001894.Misoprostol mifepristone for termination of pregnancy [Efficacy of misoprostol administration 24 hours after mifepristone for termination of pregnancy]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=3232&EncHid=&userNa... (registered on 18 July 2011).
Dabash 2012 {published data only}
-
- Dabash R, Blum J, Raghavan S, Ngoc NT, Chelli H, Hajri S, et al.Outcomes of a double-blind randomized trial comparing misoprostol-only to mifepristone+misoprostol for home-based early medical abortion. International Journal of Gynaecology and Obstetrics 2012;119:S315. - PubMed
Dalenda 2010 {published data only}
-
- Dalenda C, Ines N, Fathia B, Malika A, Bechir Z, Ezzeddine S, et al.Two medical abortion regimens for late first-trimester termination of pregnancy: a prospective randomized trial. Contraception 2010;81(4):323-7. [DOI: ] - PubMed
Davis 1999 {published data only}
-
- Davis AR, Miller L, Tamimi H, Gown A.Methotrexate compared with mercaptopurine for early induced abortion. Obstetrics and Gynecology 1999;93:904-9. - PubMed
De 2019 {published data only}
Dehbashi 2016 {published data only}
De Nonno 2000 {published data only}
-
- De Nonno LS, Westhoff C, Fielding S, Schaff E.Timing of pain and bleeding after mifepristone induced abortion. Contraception 2000;62(6):305-9. - PubMed
Derakhshan Aydenloo 2018 {published data only}
-
- Derakhshan-Aydenloo S, Behroozi-Lak T, Broomand F.Evaluation of effect of letrozole prior to misoprostol in comparison with misoprostol alone in success rate of induced abortion. Journal of Gynecology Obstetrics and Human Reproduction 2018;47(3):113-7. - PubMed
Dey 2013 {published data only}
Duhan 2011 {published data only}
-
- Duhan N, Gupta S, Dahiya K, Sirohiwal D, Rohilla S.Comparison of isosorbide mononitrate and misoprostol for cervical ripening in termination of pregnancy between 8 and 12 weeks: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(6):1245-8. [DOI: ] - PubMed
Elbareg 2018 {published data only}
-
- Elbareg A, Essadi FM.Letrozole combined with misoprostol versus (MISO) alone in management of delayed miscarriages. International Journal of Gynecology and Obstetrics 2018;143:387. [DOI: 10.1002/ijgo.12582] - DOI
ICMR 2000 {published data only}
-
- Indian Council of Medical Research Task Force.A multicentre randomized comparative clinical trial of 200 mg RU486 (mifepristone) single dose followed by either 5 mg 9-methylene PGE2 Gel (meteneprost) or 600 mcg oral PGE1 (misoprostol) for termination of early pregnancy within 28 days of missed menstrual period. Contraception 2000;62:125-30. - PubMed
IRCT201207259491N3 {published data only}
-
- IRCT201207259491N3.The effect of adding hyoscine to vaginal misoprostol on shortening the induction time of abortion. irct.ir/trial/10080 (registration date 22 August 2012).
IRCT2013090414563N1 {published data only}
-
- IRCT2013090414563N1.Efficacy of misoprostol on abortion therapy. www.irct.ir/trial/14176 (registration date 27 January 2014).
IRCT2014030114293N1 {published data only}
-
- IRCT2014030114293N1.Comparison the effect of letrozole plus misoprostol and misoprostol alone in termination of nonviable first trimester pregnancies; a single blinded randomized trial. irct.ir/trial/13912 (registration date 14 March 2014).
IRCT2014070711020N4 {published data only}
-
- IRCT2014070711020N4.Improving the effectiveness of misoprostol cervical ripening with laminaria. irct.ir/trial/11383 (registration date 24 July 2015).
IRCT2014110812789N9 {published data only}
-
- IRCT2014110812789N9.Comparison of oral versus vaginal misoprostol for legal abortion. irct.ir/trial/12797 (registration date 13 December 2014).
IRCT201412111760N37 {published data only}
-
- IRCT201412111760N37.The compression of misoprostol effect with and without letrozole in first trimester abortion: a randomized clinical trial study. irct.ir/trial/1301 (registration date 22 December 2014).
IRCT2017011526962N3 {published data only}
-
- IRCT2017011526962N3.The effect of letrozole in combination with misoprostol on induced abortion success rate. irct.ir/trial/22225 (registration date 10 February 2017).
IRCT2017040633255N1 {published data only}
-
- IRCT2017040633255N1.Comparison of the effect between two dose (800 µg/day & 400 µg/6) of vaginal misoprostol in the termination of first-trimester pregnancy: a double-blinded randomized trial. irct.ir/trial/25706 (registration date 25 June 2017).
IRCT201704179014N159 {published data only}
-
- IRCT201704179014N159.Comparison of the effect of vaginal versus sublingual misoprostol in medical induction of missed abortions. irct.ir/trial/9597 (registration date 30 April 2017).
IRCT20180109038284N1 {unpublished data only}
-
- IRCT20180109038284N1.Comparison of induction of abortion in both inpatients and outpatients in the first trimester of pregnancy [Comparison of successes and complications of inpatient and outpatient treatment in the first trimester abortion with misoprostol]. irct.ir/trial/28855 (registration date 25 June 2019).
Jacobson 1990 {published data only}
JapicCTI‐195008 {published data only}
-
- JapicCTI-195008.The phase 3 study of LPI 001 and LPI 002. rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-195008 (registered date 17 October 2019).
Lee 2011a {published data only}
-
- Lee VC, Yung SS, Li RH, Watzer B, Schweer H, Ng EH, et al.A randomized comparison of pharmacokinetics of a single vaginal dose of dry misoprostol or misoprostol moistened with normal saline or with acetic acid. Human Reproduction (Oxford, England) 2011;26(11):2981-7. [DOI: ] - PubMed
Lee 2011b {published data only}
-
- Lee VC, Tang OS, Ng EH, Yeung WS, Ho PC.A pilot study on the use of letrozole with either misoprostol or mifepristone for termination of pregnancy up to 63 days. Contraception 2011;83(1):62-7. [DOI: ] - PubMed
Lee 2012 {published data only}
-
- Lee VC, Yeung TW, Tang OS, Ng EH, Yeung WS, Ho PC.Effect of letrozole on uterine artery Doppler flow indices prior to first-trimester termination of pregnancy: a randomized controlled trial. Ultrasound in Obstetrics & Gynecology 2012;40(4):392-7. [DOI: ] - PubMed
Li 2012 {published data only}
-
- Li Cl, Chen DJ, Sheng XJ, Liu MS, Weng HN, Du PL, et al.The lowest dosages of mifepristone and misoprostol to terminate ultra-early pregnancy. Zhonghua Fu Chan Ke za Zhi 2012;47(10):764-8. - PubMed
Martin 1998 {published data only}
-
- Martin CW, Brown AH, Baird DT.A pilot study of the effect of methotrexate or combined oral contraceptive on bleeding patterns after induction of abortion with mifepristone and a prostaglandin pessary. Contraception 1998;58(2):99-103. - PubMed
Mostafa Gharebaghi 2010 {published data only}
-
- Mostafa-Gharebaghi P, Mansourfar M, Sadeghi-Bazargani H.Low dose vaginal misoprostol versus prostaglandin E2 suppository for early uterine evacuation: a randomized clinical trial. Pakistan Journal of Biological Sciences 2010;13(19):946-50. - PubMed
NCT01156688 {published data only}
-
- NCT01156688.A prospective randomized comparison trial on the use of mifepristone with sublingual or buccal misoprostol for medical abortions of less than 9 weeks gestation. clinicaltrials.gov/ct2/show/NCT01156688?term=NCT01156688&draw=2&... (first posted 5 July 2010).
NCT01173003 {published data only}
-
- NCT01173003.Expansion study of a simplified regimen of medical abortion thru 63 last menstrual period (LMP) in Tunisia. clinicaltrials.gov/ct2/show/NCT01173003?term=NCT01173003&draw=2&... (first posted 30 July 2010).
NCT01387256 {published data only}
-
- NCT01387256.Comparing two regimens for medical abortion: mifepristone + misoprostol versus misoprostol alone. clinicaltrials.gov/ct2/results?cond=&term=NCT01387256&cntry=&... (first posted 4 July 2011).
NCT01475318 {published data only}
-
- NCT01475318.Study on combined use of letrozole, mifepristone and misoprostol in termination of pregnancy. clinicaltrials.gov/ct2/show/NCT01475318?term=NCT01475318&draw=2&... (first posted 21 November 2011).
NCT01615211 {published data only}
-
- NCT01615211.Randomized study of letrozole and trilostane for medical abortion. clinicaltrials.gov/ct2/show/NCT01615211?term=NCT01615211&draw=2&... (first posted 8 June 2012).
NCT01615224 {published data only}
-
- NCT01615224.Repeated doses of misoprostol for medical treatment of missed miscarriage. clinicaltrials.gov/ct2/show/NCT01615224?term=NCT01615224&draw=2&... (first posted 8 June 2012).
NCT01811056 {published data only}
-
- NCT01811056.Exploring a patient-centered approach to mifepristone administration in medical abortion. clinicaltrials.gov/ct2/show/NCT01811056?term=NCT01811056&draw=2&... (first posted 14 March 2013).
NCT01818414 {published data only}
-
- NCT01818414.Same-day Dilapan-S with adjunctive misoprostol. clinicaltrials.gov/ct2/show/NCT01818414?term=NCT01818414&draw=2&... (first posted 26 March 2013).
NCT01827995 {published data only}
-
- NCT01827995.Simplified medical abortion in rural India. clinicaltrials.gov/ct2/show/NCT01827995?term=NCT01827995&draw=2&... (first posted 10 April 2013).
NCT01856985 {published data only}
-
- NCT01856985.Acceptability of an out-patient regimen of medical abortion with mifepristone and 800 mcg misoprostol administered at 78-84 days gestation. clinicaltrials.gov/ct2/show/NCT01856985?term=NCT01856985&draw=2&... (first posted 20 May 2013).
NCT01920022 {published data only}
-
- NCT01920022.Quickstart of Nexplanon® at medical abortion. clinicaltrials.gov/ct2/show/NCT01920022?term=NCT01920022&draw=2&... (first posted 9 August 2013).
NCT01966874 {published data only}
-
- NCT01966874.Mifepristone and misoprostol for the termination of pregnancy up to 70 days gestation. clinicaltrials.gov/ct2/show/NCT01966874?term=NCT01966874&draw=2&... (first posted 22 October 2013).
NCT02012491 {published data only}
-
- NCT02012491.Comparative effectiveness of pregnancy failure management regimens. clinicaltrials.gov/ct2/show/NCT02012491?term=NCT02012491&draw=2&... (first posted 16 December 2013).
NCT02018796 {published data only}
-
- NCT02018796.Mifepristone and misoprostol for the termination of pregnancy up to 70 days' gestation in Kazakhstan. clinicaltrials.gov/ct2/show/NCT02018796?term=NCT02018796&draw=2&... (first posted 23 December 2013).
NCT02141555 {published data only}
-
- NCT02141555.Comparing buccal and vaginal misoprostol in management of early pregnancy loss. clinicaltrials.gov/ct2/show/NCT02141555?term=NCT02141555&draw=2&... (first posted 19 May 2014).
NCT02191774 {published data only}
-
- NCT02191774.Medical abortion up to 10 weeks gestation at home. clinicaltrials.gov/ct2/show/NCT02191774?term=NCT02191774&draw=2&... (first posted 16 July 2014).
NCT02299401 {published data only}
-
- NCT02299401.Effectiveness of two regimens of misoprostol alone for early pregnancy termination and use of SQPT for at-home follow-up. clinicaltrials.gov/ct2/show/NCT02299401?term=NCT02299401&draw=2&... (first posted 24 November 2014).
NCT02314754 {published data only}
-
- NCT02314754.Outpatient medical abortion with mifepristone and misoprostol through 77 days of gestation. clinicaltrials.gov/ct2/show/NCT02314754?term=NCT02314754&draw=2&... (first posted 11 December 2014).
NCT02342002 {published data only}
-
- NCT02342002.Mifepristone and misoprostol versus misoprostol alone for missed abortion: a randomized-controlled trial. clinicaltrials.gov/ct2/show/NCT02342002?term=NCT02342002&draw=2&... (first posted 19 January 2015).
NCT02363556 {published data only}
-
- NCT02363556.Comparing misoprostol alone to dilapan with misoprostol and comparing buccal to vaginal misoprostol. clinicaltrials.gov/ct2/show/NCT02363556?term=NCT02363556&draw=2&... (first posted 16 February 2015).
NCT02401425 {published data only}
-
- NCT02401425.Use of letrozole pretreatment with misoprostol for first-trimester medical abortion. clinicaltrials.gov/ct2/show/NCT02401425?term=NCT02401425&draw=2&... (first posted 27 March 2015).
NCT02480543 {published data only}
-
- NCT02480543.Different routes of misoprostol prior to first trimester surgical abortion. clinicaltrials.gov/ct2/show/NCT02480543?term=NCT02480543&draw=2&... (first posted 24 June 2015).
NCT02515604 {published data only}
-
- NCT02515604.Comparison of single and repeated dose of vaginal misoprostol for the treatment of early pregnancy failure. clinicaltrials.gov/ct2/show/NCT02515604?term=NCT02515604&draw=2&... (first posted 5 August 2015).
NCT02522078 {published data only}
-
- NCT02522078.Dry vs wet misoprostol for cervical dilation in first trimester abortion. clinicaltrials.gov/ct2/show/NCT02522078?term=NCT02522078&draw=2&... (first posted 13 August 2015).
NCT02614781 {published data only}
-
- NCT02614781.Efficacy of mifepristone - prostaglandin analogue combination in medical termination of pregnancy beyond 7 weeks of amenorrhea. clinicaltrials.gov/ct2/show/NCT02614781?term=NCT02614781&draw=2&... (first posted 25 November 2015).
NCT02686840 {published data only}
-
- NCT02686840.Sublingual versus vaginal misoprostol in medical treatment of first trimestric missed miscarriage. clinicaltrials.gov/ct2/show/NCT02686840 (first received 22 February 2016).
NCT02707653 {published data only}
-
- NCT02707653.Sublingual misoprostol for the treatment of incomplete abortion: operations research. clinicaltrials.gov/ct2/show/NCT02707653?term=NCT02707653&draw=2&... (first posted 14 March 2016).
NCT02720991 {published data only}
-
- NCT02720991.A pilot of an outpatient regimen of medical abortion with mifepristone and sublingual misoprostol in the 11 and 12 weeks. clinicaltrials.gov/ct2/show/NCT02720991?term=NCT02720991&draw=2&... (first posted 28 March 2016).
NCT02957305 {published data only}
-
- NCT02957305.Misoprostol 400 µg versus 200 µg for cervical ripening in 1st trimester miscarriage. clinicaltrials.gov/ct2/show/NCT02957305?term=NCT02957305&draw=2&... (first posted 6 November 2016).
NCT03140384 {published data only}
-
- NCT03140384.Compare the different routes of administration of misoprostol during medicinal abortion between 7 and 9 weeks of amenorrhoea (SA). clinicaltrials.gov/ct2/show/NCT03140384?term=NCT03140384&draw=2&... (first posted 4 May 2017).
NCT03320057 {published data only}
-
- NCT03320057.Medication abortion via pharmacy dispensing. clinicaltrials.gov/ct2/show/NCT03320057?term=NCT03320057&draw=2&... (first posted 25 October 2017).
NCT03727308 {published data only}
-
- NCT03727308.A prospective, comparative study of clinical outcomes following clinic-based versus self-use of medical abortion using mifepristone with misoprostol. clinicaltrials.gov/ct2/show/NCT03727308?term=NCT03727308&draw=2&... (first posted 1 November 2018).
Ngai 2000 {published data only}
-
- Ngai SW, Tang OS, Chan YM, Ho PC.Vaginal misoprostol alone for medical abortion up to 9 weeks of gestation: efficacy and acceptability. Human Reproduction (Oxford, England) 2000;15(5):1159-62. - PubMed
NL6366 {published data only}
-
- NL6366.Comparing two medical treatments for early pregnancy failure. trialregister.nl/trial/6366 (registered date 3 July 2017).
Norman 1992 {published data only}
-
- Norman JE, Thong KJ, Rodger MW, Baird DT.Medical abortion in women of <56 days amenorrhoea: a comparison between gemeprost (a PGE1 analogue) alone and mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1992;99:601-6. - PubMed
Parveen 2011 {published data only}
Prabhu 2015 {published data only}
-
- Prabhu S, Priyanka HK, Shetty A.A study of different doses of sublingual misoprostol after oral mifepristone in medical termination of pregnancy. Journal of Evolution of Medical and Dental Sciences 2015;4(91):15673-8.
Prine 2010 {published data only}
-
- Prine L, Shannon C, Gillespie G, Crowden WA, Fortin J, Howe M, et al.Medical abortion: outcomes in a family medicine setting. Journal of the American Board of Family Medicine 2010;23(4):509-13. [DOI: ] - PubMed
Rezai 2014 {published data only}
-
- Rezai Z, Heydari Bazardehi SS, Ghasemi Nezhad A, Saeid Sadeghi A, Ghorbani Yekta B.Letrozole and misoprostol versus misoprostol alone for termination of pregnancy: a randomized clinical trial. Tehran University Medical Journal 2014;71(11):700-6.
Rouzi 2014 {published data only}
Seervi 2014 {published data only}
Swahn 1994 {published data only}
-
- Swahn ML, Kovacs L, Cekan SZ, Aedo AR, Westlund P.Termination of early pregnancy with ZK 98,734: pharmacokinetic behaviour and clinical effect. Human Reproduction (Oxford, England) 1994;9(1):57-63. - PubMed
Swica 2013 {published data only}
-
- Swica Y, Chong E, Middleton T, Prine L, Gold M, Schreiber CA, et al.Acceptability of home use of mifepristone for medical abortion. Contraception 2013;88(1):122-7. [DOI: ] - PubMed
Tang 1999 {published data only}
-
- Tang OS, Gao PP, Cheng L, Lee SW, Ho PC.A randomized double-blind placebo-controlled study to assess the effect of oral contraceptive pills on the outcome of medical abortion with mifepristone and misoprostol. Human Reproduction (Oxford, England) 1999;14(3):722-5. - PubMed
Tehranian 2010 {published data only}
-
- Tehranian A, Beigishah F, Moini A, Arab M, Farzaneh F.The effect of adding hyoscine to vaginal misoprostol on abortion induction success rate. Tehran University Medical Journal 2010;68(4):220-5.
Torre 2012 {published data only}
-
- Torre A, Huchon C, Bussieres L, Machevin E, Camus E, Fauconnier A.Immediate versus delayed medical treatment for first-trimester miscarriage: a randomized trial. American Journal of Obstetrics and Gynecology 2012;206(3):215.e1-6. [DOI: ] - PubMed
Tsereteli 2010 {published data only}
-
- Tsereteli T, Ngoc NT, Chong E, Winikoff B.Reducing the buccal dose of misoprostol in mifepristone medical abortion up to 63 days LMP. In: The European Journal of Contraception & Reproductive Health Care. Vol. 15. 2010:36.
Varona Sanchez 2010 {published data only}
-
- Varona Sanchez JA, Borrego Lopez JA, Formoso Martin LE, Martinez Martinez-Pinillo A.Application of misoprostol in adolescent early pregnancy [[Misoprostol en la interrupcin temprana del embarazo en pacientes adolescentesies]]. Revista Cubana de Obstetricia y Ginecologia 2010;36(1):97-108.
Wedisinghe 2010 {published data only}
-
- Wedisinghe L, Elsandabesee D.Flexible mifepristone and misoprostol administration interval for first-trimester medical termination. Contraception 2010;81(4):269-74. [DOI: ] - PubMed
Wen 2010 {published data only}
-
- Wen SM, Xiao Q, Ma XD.Clinical application of mifepristone and misoprostol of different administration methods in termination of pregnancy in 160 pregnant women in the first trimester pregnancy. Maternal and Child Health Care of China 2010;25(16):2239-41.
Wiebe 2001 {published data only}
-
- Wiebe ER.Misoprostol administration in medical abortion. A comparison of 3 regimens. Journal of Reproductive Medicine 2001;46(2):125-9. - PubMed
Winikoff 2012 {published data only}
-
- Winikoff B, Dzuba IG, Chong E, Goldberg AB, Lichtenberg ES, Ball C, et al.Extending outpatient medical abortion services through 70 days of gestational age. Obstetrics and Gynecology 2012;120(5):1070-6. [DOI: ] - PubMed
Yung 2016 {published data only}
-
- Yung SS, Lee VC, Chiu PC, Li HW, Ng EH, Yeung WS, et al.The effect of 7 days of letrozole pretreatment combined with misoprostol on the expression of progesterone receptor and apoptotic factors of placental and decidual tissues from first-trimester abortion: a randomized controlled trial. Contraception 2016;93(4):323-30. [DOI: ] - PubMed
References to studies awaiting assessment
Ayati 2013 {published data only}
-
- Ayati S, Roudsari FV, Banavi M, Shakeri MT, Berahmat A.Comparison between rectal misoprostol and vaginal misoprostol for first trimester termination of pregnancy in patients with previous uterus surgery. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;15(42):Pe1-6.
Ibrahim 2019 {published data only}
-
- Ibrahim HN, Mustafa ZM, Rajab HK.Comparison between the effectiveness of combination of letrozole with misoprostol and tamoxifen with misoprostol in medical termination of first trimester missed miscarriage. Indian Journal of Public Health Research and Development 2019;10(8):2322.
IRCT201403244686N11 {unpublished data only}
-
- IRCT201403244686N11.The comparison of the efficacy of oral castor oil with vaginally misoprostol and vaginally misoprostol alone in the treatment of missed abortion. irct.ir/trial/5024 (registration date 13 April 2014).
IRCT20150407021653N19 {unpublished data only}
-
- IRCT20150407021653N19.An outpatient treatment of comparative study on the efficacy and side effects of oxytocin + misoprostol with methyl ergonovin (metergin) + misoprostol and only misoprostol in the expulsion of first trimester intrauterine fetal dead. irct.ir/trial/34519 (registration date 4 October 2019).
IRCT2015112421506N3 {unpublished data only}
-
- IRCT2015112421506N3.INH plus misoprostol vs. misoprostol alone for early medical abortion: clinical trial. irct.ir/trial/18840 (registration date 1 February 2016).
IRCT2017042533635N1 {unpublished data only}
-
- IRCT2017042533635N1.Comparison the effect of rectal and oral misoprostol in first trimester of pregnancy Induced abortion. irct.ir/trial/25896 (registration date 21 June 2017).
IRCT2017070934981N1 {unpublished data only}
-
- IRCT2017070934981N1.Comparison of induction of abortion in the first trimester using misoprostol and misoprostol with estrogen priming. irct.ir/trial/26580 (registration date 16 July 2017).
Jha 2013 {published data only}
-
- Jha T, Das A, Bhattacharya AR, Ganguly RP, Patra KK, Das B.Comparison of two doses of oral misoprostol with one, after mifepristone in early abortion. Journal of the Indian Medical Association 2013;111(12):826-8. - PubMed
NCT01508143 {unpublished data only}
-
- NCT01508143.Comparitive study of two misoprostol regimen in early pregnancy termination. clinicaltrials.gov/ct2/show/NCT01508143?term=NCT01508143&draw=2&... (registration date 11 January 2012).
NCT03487354 {published and unpublished data}
-
- NCT03487354.Medical abortion with multiple vs single daily dose of misoprostol in first trimester miscarriage: a randomized clinical trial.. clinicaltrials.gov/ct2/show/NCT03487354 (first posted 4 April 2018).
References to ongoing studies
CTRI201606006980 {published data only}
-
- CTRI/2016/06/006980.Medicines for abortion between 10-20 weeks. ctri.nic.in/Clinicaltrials/showallp.php?mid1=8226&EncHid=&userNa... (registration date 2 June 2016).
EUCTR2017‐004083‐35‐FR {published data only}
-
- EUCTR2017-004083-35-FR.A double-blind, randomized, multicenter study evaluating 200 mg versus 600 mg of mifepristone on pain in voluntary abortion by drug prior to 7 SA. DoMy Study. clinicaltrialsregister.eu/ctr-search/trial/2017-004083-35/FR (registration date 9 February 2018).
EUCTR2018‐003675‐35‐SE {published data only}
-
- EUCTR2018-003675-35-SE.Medical abortion in very early pregnancy. clinicaltrialsregister.eu/ctr-search/trial/2018-003675-35/SE (registration date 24 September 2018).
NCT02704481 {published data only}
-
- NCT02704481.Non-surgical alternatives to treatment of failed medical abortion. clinicaltrials.gov/ct2/show/NCT02704481?term=NCT02704481&draw=2&... (first posted 10 March 2016).
NCT03065660 {published data only}
-
- NCT03065660.Mifepristone and misoprostol versus misoprostol alone in the medical management of missed miscarriage. clinicaltrials.gov/ct2/show/NCT03065660?term=NCT03065660&draw=2&... (first posted 28 February 2017).
NCT03440866 {published data only}
-
- NCT03440866.Comparison of the effectiveness of treatment with mifepristone and misoprostol at the same time compared to the administration of drugs at a 48-hour interval for medical abortion. clinicaltrials.gov/ct2/show/NCT03440866?term=NCT03440866&draw=2&... (first posted 22 February 2018).
NCT03628625 {published data only}
-
- NCT03628625.Letrozole pretreatment with misoprostol induction of abortion in first-trimester missed miscarriage. clinicaltrials.gov/ct2/show/NCT03628625?term=NCT03628625&draw=2&... (first posted 14 August 2018).
NCT03989869 {published data only}
-
- NCT03989869.Very early medical abortion. clinicaltrials.gov/ct2/show/NCT03989869?term=NCT03989869&draw=2&... (first posted 18 June 2019).
NCT04215835 {published data only}
-
- NCT04215835.Letrozole pretreatment with misoprostol induction of abortion in first-trimester missed miscarriage. clinicaltrials.gov/ct2/show/NCT04215835 (first received 2 January 2020).
TCTR20180825005 {published data only}
-
- TCTR20180825005.Comparison efficacy for termination of early pregnancy failure by mifepristone followed by misoprostol with misoprostol alone: a randomized double-blind placebo- controlled trial. thaiclinicaltrials.org/show/TCTR20180825005 (first posted 25 August 2018).
Van den Berg 2019 {published data only}
-
- Van den Berg J, Hamel CC, Snijders MP, Coppus SF, Vandenbussche FP.Mifepristone and misoprostol versus misoprostol alone for uterine evacuation after early pregnancy failure: study protocol for a randomized double blinded placebo controlled comparison (Triple M Trial). BMC Pregnancy and Childbirth 2019;19(1):443. [DOI: 10.1186/s12884-019-2497-y] - DOI - PMC - PubMed
Additional references
Abubeker 2020
Al Wattar 2019
-
- Al Wattar BH, Murugesu N, Tobias A, Zamora J, Khan KS.Management of first-trimester miscarriage: a systematic review and network meta-analysis. Human Reproduction Update 2019;25(3):362-74. - PubMed
Bachelot 1992
-
- Bachelot A, Cludy L, Spira A.Conditions for choosing between drug-induced and surgical abortions. Contraception 1992;45:547-59. - PubMed
Baiju 2019
-
- Baiju N, Acharya G, D'Antonio F, Berg RC.Effectiveness, safety and acceptability of self-assessment of the outcome of first-trimester medical abortion: a systematic review and meta-analysis. BJOG 2019;126(13):1536-44. - PubMed
Barnard 2015
Beaulieu 1997
Blanchard 1999
-
- Blanchard K, Winikoff B, Ellertson C.Misoprostol used alone for the termination of early pregnancy. Contraception 1999;59:209-17. - PubMed
Bugalho 1996
-
- Bugalho A, Faundes A, Jamisse L, Usfa M, Maria E, Bique C.Evaluation of the effectiveness of vaginal misoprostol to induce first trimester abortion. Contraception 1996;53:243-6. - PubMed
Bygdeman 1985
-
- Bygdeman M, Swahn ML.Progesterone receptor blockage. Effect on uterine contractility and early pregnancy. Contraception 1985;32:45-51. - PubMed
Bygdeman 2002
-
- Bygdeman M, Danielsson KG.Options for early therapeutic abortion. Drugs 2002;62(17):2459-70. - PubMed
Carbonell 1997a
-
- Carbonell JL, Varela L, Velazco A, Fernandez C.The use of misoprostol for termination of early pregnancy. Contraception 1997;55:165-8. - PubMed
Chen 2015b
-
- Chen MJ, Creinin MD.Mifepristone with buccal misoprostol for medical abortion: a systematic review. Obstetrics and Gynecology 2015;126(1):12-21. - PubMed
Costa 1998
-
- Costa SH.Commercial availability of misoprostol and induced abortion in Brazil. International Journal of Gynaecology and Obstetrics 1998;63 Suppl 1:S:131-9. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence.Version accessed 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Creinin 1993
-
- Creinin MD, Darney PD.Methotrexate and misoprostol for early abortion. Contraception 1993;48:339-48. - PubMed
Creinin 1996
-
- Creinin MD, Burke AE.Methotrexate and misoprostol for early abortion: a multicenter trial. Acceptability. Contraception 1996;54:19-22. - PubMed
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Version accessed 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grimes 1997
-
- Grimes DA.Medical abortion in early pregnancy: a review of the evidence. Obstetrics and Gynecology 1997;89:790-6. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/PDF/v5.2/chapter-08.
Honkanen 2002
-
- Honkanen H, Hertzen H.Users' perspectives on medical abortion in Finland. Contraception 2002;65(6):419-23. - PubMed
Kapp 2018
Kim 2017
Li 2021
-
- Li T, Higgins JP, Deeks JJ, editor(s).Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Nash 2018
-
- Nash CM, Philp L, Shah P, Murphy KE.Letrozole pretreatment prior to medical termination of pregnancy: a systematic review. Contraception 2018;97(6):504-9. - PubMed
Page 2021
Raymond 2013
-
- Raymond EG, Shannon C, Weaver MA, Winikoff B.First-trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception 2013;87(1):26-37. - PubMed
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Say 2010
Schmidt‐Hansen 2020
-
- Schmidt-Hansen M, Pandey A, Lohr PA, Nevill M, Taylor P, Hasler E, et al.Expulsion at home for early medical abortion: a systematic review with meta-analyses. Acta Obstetricia et Gynecologica Scandinavica 2020;100(4):727-35. [CENTRAL: 10.1111/aogs.14025] - PubMed
Sedgh 2016
Sjöström 2016
Sjöström 2017
Urquhart 1997
-
- Urquhart DR, Templeton AA, Shinewi F, Chapman M, Hawkins K, McGarry J.The efficacy and tolerance of mifepristone and prostaglandin in termination of pregnancy of less than 63 days gestation: UK multicentre study - final results. Contraception 1997;55:1-5. - PubMed
WHO 1997
-
- WHO Scientific Group.Medical methods for termination of pregnancy. Report of a WHO Scientific Group. WHO Technical Report Series. WHO edition. Vol. 871. Geneva: WHO, 1997. - PubMed
Winikoff 1997
-
- Winikoff B, Irving S, Coyaji K, Caberas E, Bilian X, Sujuan G, et al.Safety, efficacy and acceptability of medical abortion in China, Cuba and India: a comparative trial of mifepristone - misoprostol versus surgical abortion. American Journal of Obstetrics and Gynecology 1997;176:431-7. - PubMed
Yeung 2012
-
- Yeung TW, Lee VC, Ng EH, Ho PC.A pilot study on the use of a 7-day course of letrozole followed by misoprostol for the termination of early pregnancy up to 63 days. Contraception 2012;86:763-9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

