Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance
- PMID: 35608633
- PMCID: PMC9614730
- DOI: 10.1021/acs.chemrev.2c00021
Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance
Abstract
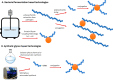
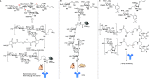
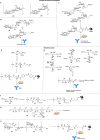
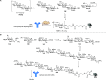
Antimicrobial resistance (AMR) is emerging as the next potential pandemic. Different microorganisms, including the bacteria Acinetobacter baumannii, Clostridioides difficile, Escherichia coli, Enterococcus faecium, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, non-typhoidal Salmonella, and Staphylococcus aureus, and the fungus Candida auris, have been identified by the WHO and CDC as urgent or serious AMR threats. Others, such as group A and B Streptococci, are classified as concerning threats. Glycoconjugate vaccines have been demonstrated to be an efficacious and cost-effective measure to combat infections against Haemophilus influenzae, Neisseria meningitis, Streptococcus pneumoniae, and, more recently, Salmonella typhi. Recent times have seen enormous progress in methodologies for the assembly of complex glycans and glycoconjugates, with developments in synthetic, chemoenzymatic, and glycoengineering methodologies. This review analyzes the advancement of glycoconjugate vaccines based on synthetic carbohydrates to improve existing vaccines and identify novel candidates to combat AMR. Through this literature survey we built an overview of structure-immunogenicity relationships from available data and identify gaps and areas for further research to better exploit the peculiar role of carbohydrates as vaccine targets and create the next generation of synthetic carbohydrate-based vaccines.
Conflict of interest statement
The authors declare the following competing financial interest(s): L.D.B., F.N., M.R.R., and R.A. are employees of the GSK group of companies. Bexsero is a trademark from GSK. Trumemeba is a trademark of Pfizer. QumiHib is a trademark of CIGB.
Figures















References
-
- Peltola H.; Mäkelä H.; Käyhty H.; Jousimies H.; Herva E.; Hällström K.; Sivonen A.; Renkonen O. V.; Pettay O.; Karanko V.; et al. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N. Engl. J. Med. 1977, 297, 686–691. 10.1056/NEJM197709292971302. - DOI - PubMed
-
- Stacey H. L.; Rosen J.; Peterson J. T.; Williams-Diaz A.; Gakhar V.; Sterling T. M.; Acosta C. J.; Nolan K. M.; Li J.; Pedley A.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum. Vaccin. Immunother. 2019, 530–539. 10.1080/21645515.2018.1532249. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

