Flow-controlled ventilation in moderate acute respiratory distress syndrome due to COVID-19: an open-label repeated-measures controlled trial
- PMID: 35608696
- PMCID: PMC9127816
- DOI: 10.1186/s40635-022-00449-4
Flow-controlled ventilation in moderate acute respiratory distress syndrome due to COVID-19: an open-label repeated-measures controlled trial
Abstract
Background: Flow-controlled ventilation (FCV), a novel mode of mechanical ventilation characterised by constant flow during active expiration, may result in more efficient alveolar gas exchange, better lung recruitment and might be useful in limiting ventilator-induced lung injury. However, data regarding FCV in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome (ARDS) are scarce.
Objectives: We hypothesised that the use of FCV is feasible and would improve oxygenation in moderate COVID-19 ARDS compared to conventional ventilation.
Design: Open-label repeated-measures controlled trial.
Setting: From February to April 2021, patients with moderate COVID-19 ARDS were recruited in a tertiary referral intensive care unit.
Patients: Patients with moderate ARDS (PaO2/FIO2 ratio 100-200 mmHg, SpO2 88-94% and PaO2 60-80 mmHg) were considered eligible. Exclusion criteria were: extremes of age (< 18 years, > 80 years), obesity (body mass index > 40 kg/m2), prone positioning at the time of intervention, mechanical ventilation for more than 10 days and extracorporeal membrane oxygenation. Eleven patients were recruited.
Intervention: Participants were ventilated in FCV mode for 30 min, and subsequently in volume-control mode (VCV) for 30 min.
Main outcome measures: Feasibility of FCV to maintain oxygenation was assessed by the PaO2/FiO2 ratio (mmHg) as a primary outcome parameter. Secondary outcomes included ventilator parameters, PaCO2 and haemodynamic data. All adverse events were recorded.
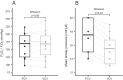
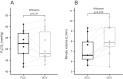
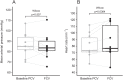
Results: FCV was feasible in all patients and no adverse events were observed. There was no difference in the PaO2/FIO2 ratio after 30 min of ventilation in FCV mode (169 mmHg) compared to 30 min of ventilation in VCV mode subsequently (168 mmHg, 95% CI of pseudo-medians (- 10.5, 3.6), p = 0.56). The tidal volumes (p < 0.01) and minute ventilation were lower during FCV (p = 0.01) while PaCO2 was similar at the end of the 30-min ventilation periods (p = 0.31). Mean arterial pressure during FCV was comparable to baseline.
Conclusions: Thirty minutes of FCV in patients with moderate COVID-19 ARDS receiving neuromuscular blocking agents resulted in similar oxygenation, compared to VCV. FCV was feasible and did not result in adverse events.
Trial registration: Clinicaltrials.gov identifier: NCT04894214.
Keywords: ARDS; COVID-19; Flow-controlled ventilation; Mechanical ventilation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. - DOI - PubMed
-
- Cavalcanti AB, Suzumura ÉA, Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

