Dengue Vaccines: An Update
- PMID: 35608749
- PMCID: PMC9127483
- DOI: 10.1007/s40259-022-00531-z
Dengue Vaccines: An Update
Abstract
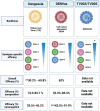
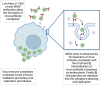
Dengue is one of the most prevalent mosquito-borne diseases in the world, affecting an estimated 390 million people each year, according to models. For the last two decades, efforts to develop safe and effective vaccines to prevent dengue virus (DENV) infections have faced several challenges, mostly related to the complexity of conducting long-term studies to evaluate vaccine efficacy and safety to rule out the risk of vaccine-induced DHS/DSS, particularly in children. At least seven DENV vaccines have undergone different phases of clinical trials; however, only three of them (Dengvaxia®, TV003, and TAK-003) have showed promising results, and are addressed in detail in this review in terms of their molecular design, efficacy, and immunogenicity. Safety-related challenges during DENV vaccine development are also discussed.
© 2022. The Author(s).
Conflict of interest statement
Jesús M. Torres-Flores, Arturo Reyes-Sandoval, and Ma. Isabel Salazar have no conflicts of interest to declare.
Figures

References
-
- Deming R, Manrique-Saide P, Medina Barreiro A, Cardeña EU, Che-Mendoza A, Jones B, Liebman K, et al. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasit Vectors. 2016;9:67. doi: 10.1186/s13071-016-1346-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

