Selective Pharmacologic Therapies for Dry Eye Disease Treatment: Efficacy, Tolerability, and Safety Data Review from Preclinical Studies and Pivotal Trials
- PMID: 35608780
- PMCID: PMC9253213
- DOI: 10.1007/s40123-022-00516-9
Selective Pharmacologic Therapies for Dry Eye Disease Treatment: Efficacy, Tolerability, and Safety Data Review from Preclinical Studies and Pivotal Trials
Abstract
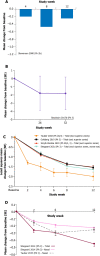
Keratoconjunctivitis sicca, also known as dry eye disease (DED), is a prevalent, multifactorial disease associated with compromised ocular lubrication, ocular surface inflammation and damage, and ocular symptoms. Several anti-inflammatory, topical ophthalmic therapies are available to treat clinical signs and symptoms of DED in the USA and Europe. Cyclosporine A (CsA)-based formulations include an ophthalmic emulsion of 0.05% CsA (CsA 0.05%), a cationic emulsion (CE) of CsA 0.1% (CsA CE), and an aqueous nanomicellar formulation of 0.09% CsA (OTX-101). Lifitegrast is a 5% ophthalmic solution of a lymphocyte function-associated antigen 1 antagonist that is believed to target T cell activation and recruitment to inhibit ocular inflammation. Here we provide a comprehensive review summarising preclinical studies and pivotal trial data for these treatments to provide a complete understanding of their efficacy and safety profile. Overall, data in the evaluated studies show a favourable risk-benefit profile for the use of targeted topical anti-inflammatory pharmacologic treatments in patients with DED. Pivotal trials for CsA 0.05%, CsA CE, OTX-101, and lifitegrast clearly demonstrate treatment efficacy compared to vehicle across treatments with no serious ocular treatment-emergent adverse events (TEAEs). Patients using ophthalmic treatments reported ocular TEAEs more frequently than those treated with vehicle; however, relatively few TEAEs led to treatment discontinuation. The specific signs and symptoms of DED that improve with treatment vary with the treatment prescribed. Long-term and direct comparative studies between treatments are needed to further understand treatment differences in efficacy and safety profiles.
Keywords: Cyclosporine A; Dry eye; Keratoconjunctivitis sicca; Lifitegrast; OTX-101; Ocular drug therapy; Ocular inflammation; Tear deficiency.
© 2022. The Author(s).
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

