Electrochemical Depolymerization of Lignin in a Biomass-based Solvent
- PMID: 35608798
- PMCID: PMC9545899
- DOI: 10.1002/cssc.202200718
Electrochemical Depolymerization of Lignin in a Biomass-based Solvent
Abstract
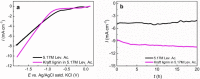
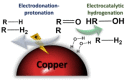
Breaking down lignin into smaller units is the key to generate high value-added products. Nevertheless, dissolving this complex plant polyphenol in an environment-friendly way is often a challenge. Levulinic acid, which is formed during the hydrothermal processing of lignocellulosic biomass, has been shown to efficiently dissolve lignin. Herein, levulinic acid was evaluated as a medium for the reductive electrochemical depolymerization of the lignin macromolecule. Copper was chosen as the electrocatalyst due to the economic feasibility and low activity towards the hydrogen evolution reaction. After depolymerization, high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy revealed lignin-derived monomers and dimers. A predominance of aryl ether and phenolic groups was observed. Depolymerized lignin was further evaluated as an anti-corrosion coating, revealing enhancements on the electrochemical stability of the metal. Via a simple depolymerization process of biomass waste in a biomass-based solvent, a straightforward approach to produce high value-added compounds or tailored biobased materials was demonstrated.
Keywords: coating; depolymerization; electrocatalysis; levulinic acid; lignin.
© 2022 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Kang S., Miao R., Guo J., Fu J., Catal. Today 2021, 374, 61–76.
-
- Kim H., Lee S., Ahn Y., Lee J., Won W., ACS Sustainable Chem. Eng. 2020, 8, 12419–12429.
-
- Zhou S.-J., Wang H.-M., Xiong S.-J., Sun J.-M., Wang Y.-Y., Yu S., Sun Z., Wen J.-L., Yuan T.-Q., ACS Sustainable Chem. Eng. 2021, 9, 12017–12042.
-
- Ashokkumar V., Venkatkarthick R., Jayashree S., Chuetor S., Dharmaraj S., Kumar G., Chen W.-H., Ngamcharussrivichai C., Bioresour. Technol. 2022, 344, 126195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

