Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis
- PMID: 35608910
- PMCID: PMC9246394
- DOI: 10.1172/JCI155350
Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis
Abstract
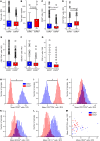
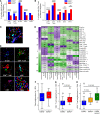
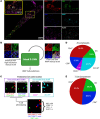
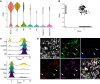
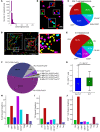
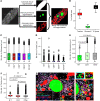
BACKGROUNDIn human lupus nephritis (LN), tubulointerstitial inflammation (TII) on biopsy predicts progression to end-stage renal disease (ESRD). However, only about half of patients with moderate-to-severe TII develop ESRD. We hypothesized that this heterogeneity in outcome reflects different underlying inflammatory states. Therefore, we interrogated renal biopsies from LN longitudinal and cross-sectional cohorts.METHODSData were acquired using conventional and highly multiplexed confocal microscopy. To accurately segment cells across whole biopsies, and to understand their spatial relationships, we developed computational pipelines by training and implementing several deep-learning models and other computer vision techniques.RESULTSHigh B cell densities were associated with protection from ESRD. In contrast, high densities of CD8+, γδ, and other CD4-CD8- T cells were associated with both acute renal failure and progression to ESRD. B cells were often organized into large periglomerular neighborhoods with Tfh cells, while CD4- T cells formed small neighborhoods in the tubulointerstitium, with frequency that predicted progression to ESRD.CONCLUSIONThese data reveal that specific in situ inflammatory states are associated with refractory and progressive renal disease.FUNDINGThis study was funded by the NIH Autoimmunity Centers of Excellence (AI082724), Department of Defense (LRI180083), Alliance for Lupus Research, and NIH awards (S10-OD025081, S10-RR021039, and P30-CA14599).
Keywords: Adaptive immunity; Autoimmunity; Lupus.
Conflict of interest statement
Figures


Comment in
-
Lymphocytes in the neighborhood: good or bad for the kidney?J Clin Invest. 2022 Jul 1;132(13):e160657. doi: 10.1172/JCI160657. J Clin Invest. 2022. PMID: 35775489 Free PMC article.
Similar articles
-
Tubulointerstitial damage predicts end stage renal disease in lupus nephritis with preserved to moderately impaired renal function: A retrospective cohort study.Semin Arthritis Rheum. 2018 Feb;47(4):545-551. doi: 10.1016/j.semarthrit.2017.07.007. Epub 2017 Jul 14. Semin Arthritis Rheum. 2018. PMID: 28803673 Free PMC article.
-
Prognostic significance of repeat biopsy in lupus nephritis: Histopathologic worsening and a short time between biopsies is associated with significantly increased risk for end stage renal disease and death.Clin Immunol. 2017 Dec;185:3-9. doi: 10.1016/j.clim.2016.11.019. Epub 2016 Dec 3. Clin Immunol. 2017. PMID: 27923701 Free PMC article.
-
Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis.Arthritis Rheumatol. 2014 Dec;66(12):3359-70. doi: 10.1002/art.38888. Arthritis Rheumatol. 2014. PMID: 25306868 Free PMC article.
-
The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis.Semin Nephrol. 2015 Sep;35(5):455-64. doi: 10.1016/j.semnephrol.2015.08.007. Semin Nephrol. 2015. PMID: 26573548 Free PMC article. Review.
-
Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression.Lupus. 2020 Aug;29(9):1011-1020. doi: 10.1177/0961203320932219. Epub 2020 Jun 22. Lupus. 2020. PMID: 32571142 Free PMC article. Review.
Cited by
-
Anthocyanin improves kidney function in diabetic kidney disease by regulating amino acid metabolism.J Transl Med. 2022 Nov 5;20(1):510. doi: 10.1186/s12967-022-03717-9. J Transl Med. 2022. PMID: 36335368 Free PMC article.
-
The Role of New 3D Pathology and Lymphocyte Expression of Interstitial Inflammation in Pediatric-Onset Lupus Nephritis.Int J Mol Sci. 2023 Feb 9;24(4):3512. doi: 10.3390/ijms24043512. Int J Mol Sci. 2023. PMID: 36834923 Free PMC article.
-
Pathogenic cellular and molecular mediators in lupus nephritis.Nat Rev Nephrol. 2023 Aug;19(8):491-508. doi: 10.1038/s41581-023-00722-z. Epub 2023 May 24. Nat Rev Nephrol. 2023. PMID: 37225921 Review.
-
Lupus Nephritis: Immune Cells and the Kidney Microenvironment.Kidney360. 2024 Sep 1;5(9):1394-1401. doi: 10.34067/KID.0000000000000531. Epub 2024 Aug 9. Kidney360. 2024. PMID: 39120952 Free PMC article. Review.
-
The immunoregulatory roles of non-haematopoietic cells in the kidney.Nat Rev Nephrol. 2024 Apr;20(4):206-217. doi: 10.1038/s41581-023-00786-x. Epub 2023 Nov 20. Nat Rev Nephrol. 2024. PMID: 37985868 Free PMC article. Review.
References
-
- Tan EM, Kunkel HG. Characteristics of a soluble nuclear antigen precipitation with sera of patients with systemic lupus erythematosus. J Immunol. 1966;96(3):464–471. - PubMed
-
- Dubois EL, ed. Lupus Erythematosus. 2 ed. W.B Saunders; 1974.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

