Peptide 17 alleviates early hypertensive renal injury by regulating the Hippo/YAP signalling pathway
- PMID: 35608936
- PMCID: PMC9544900
- DOI: 10.1111/nep.14066
Peptide 17 alleviates early hypertensive renal injury by regulating the Hippo/YAP signalling pathway
Abstract
Aim: Hypertensive nephropathy is embodied by kidney tissue fibrosis and glomerular sclerosis, as well as renal inflammation. The Hippo/YAP (yes-associated protein, YAP) axis has been reported to promote inflammation and fibrosis and may participate in the pathogenesis of heart, vascular and renal injuries. However, the role of the Hippo/YAP pathway in hypertensive renal injury has not been reported so far. We explored the role of the Hippo/YAP signalling pathway in hypertensive renal injury and the effect of peptide 17 on its effects.
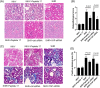
Methods: Histopathological analyses were performed based on the Masson and Haematoxylin/eosin (HE) staining approaches. Biochemical indexes were determined and immunofluorescence and western blotting were used to detect protein expression levels. The mRNA expression levels were determined by qRT-PCR.
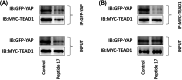
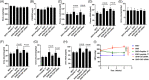
Results: Our results showed that peptide 17 reduced the systolic blood pressure (SBP) and urine protein/creatinine ratio in hypertensive rats. In addition, peptide 17 reduced the histopathological damage of kidneys in spontaneously hypertensive rats (SHRs). Moreover, peptide 17 downregulated genes in the Hippo/Yap pathway in kidney tissue of SHRs and Ang II-treated kidney cells. The expression levels of inflammatory factors TNF-α, IL-1β and MCP-1 and the pro-fibrotic factors TGF-β1, fibronectin, and CTGF were increased in the kidney of hypertensive rats, but reversed by peptide 17 treatment. Silencing of YAP had effect similar to that of peptide 17 in vivo and in vitro.
Conclusion: Peptide 17 alleviates early renal injury in hypertension by regulating the Hippo/YAP signalling pathway. These findings may be useful in the treatment of hypertensive renal injury.
Keywords: angiotensin II; hippo/YAP pathway; hypertension; peptide 17; renal damage.
© 2022 The Authors. Nephrology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Nephrology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Ruiz‐Ortega M, Rayego‐Mateos S, Lamas S, Ortiz A, Rodrigues‐Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269‐288. - PubMed
-
- Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol. 2017;956:307‐325. - PubMed
-
- Hirsch S, Hirsch J, Bhatt U, Rovin BH. Tolerating increases in the serum creatinine following aggressive treatment of chronic kidney disease, hypertension and proteinuria: pre‐renal success. Am J Nephrol. 2012;36:430‐437. - PubMed
-
- Udani S, Lazich I, Bakris GL. Epidemiology of hypertensive kidney disease. Nat Rev Nephrol. 2011;7:11‐21. - PubMed
-
- Hall JE, Granger JP, do Carmo JM, et al. Hypertension: physiology and pathophysiology. Comprehensive Phys Ther. 2012;2:2393‐2442. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

