Aberrant Multiciliogenesis in Idiopathic Pulmonary Fibrosis
- PMID: 35608953
- PMCID: PMC9348560
- DOI: 10.1165/rcmb.2021-0554OC
Aberrant Multiciliogenesis in Idiopathic Pulmonary Fibrosis
Abstract
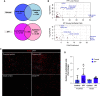
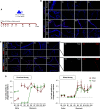
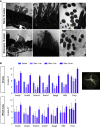
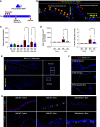
We previously identified a novel molecular subtype of idiopathic pulmonary fibrosis (IPF) defined by increased expression of cilium-associated genes, airway mucin gene MUC5B, and KRT5 marker of basal cell airway progenitors. Here we show the association of MUC5B and cilia gene expression in human IPF airway epithelial cells, providing further rationale for examining the role of cilium genes in the pathogenesis of IPF. We demonstrate increased multiciliogenesis and changes in motile cilia structure of multiciliated cells both in IPF and bleomycin lung fibrosis models. Importantly, conditional deletion of a cilium gene, Ift88 (intraflagellar transport 88), in Krt5 basal cells reduces Krt5 pod formation and lung fibrosis, whereas no changes are observed in Ift88 conditional deletion in club cell progenitors. Our findings indicate that aberrant injury-activated primary ciliogenesis and Hedgehog signaling may play a causative role in Krt5 pod formation, which leads to aberrant multiciliogenesis and lung fibrosis. This implies that modulating cilium gene expression in Krt5 cell progenitors is a potential therapeutic target for IPF.
Keywords: Ift88; Krt5 cell; abnormal structure of multiciliated cells; injury-induced multiciliogenesis.
Figures


Comment in
-
Aberrant Multiciliogenesis in Pulmonary Fibrosis: Bystander or Driver of Disease Progression?Am J Respir Cell Mol Biol. 2022 Aug;67(2):142-144. doi: 10.1165/rcmb.2022-0201ED. Am J Respir Cell Mol Biol. 2022. PMID: 35612966 Free PMC article. No abstract available.
References
-
- Boucher RC. Idiopathic pulmonary fibrosis--a sticky business. N Engl J Med . 2011;364:1560–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

