Information about dissemination of trial results in patient information leaflets for clinicals trials in the UK and Ireland: The what and the when
- PMID: 35609047
- PMCID: PMC9129017
- DOI: 10.1371/journal.pone.0268898
Information about dissemination of trial results in patient information leaflets for clinicals trials in the UK and Ireland: The what and the when
Abstract
Introduction: Complete and understandable information is vital for informed consent and this includes how and when potential participants can expect to receive trial results. Informing participants during informed consent about the sharing of trial results is important for addressing participants' needs, ensuring adherence to regulatory guidance, and in fulfilling a moral obligation.
Methods: Patient Information Leaflets (PILs) were collated from across the UK and Ireland. Trial characteristics and data on disseminating trial results was extracted. Analysis included descriptive statistics and a directed content analysis approach. The content analysis framework was informed by regulatory guidance on PIL content and existing research on dissemination of trial results. Results were analysed using descriptive statistics and presented as a narrative summary as appropriate.
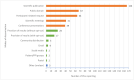
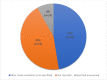
Results: 238 PILs from 178 trials were analysed. Of the 238 PILs, 74% (n = 176) provided information on sharing results with participants, 70% (n = 123) of which described passive methods of disseminating results that require active engagement from the trial participants, i.e., effort required by the participant to seek the results. The majority (90%) of PILs included more than one proposed mode of dissemination that largely targeted healthcare professionals rather than participants. Only 8% of PILs specified a time period for when results could be expected, 47% did not specify a time period (e.g. at end of trial), and 45% included no information on when trial results would be available.
Conclusion: This study found that majority of the PILs included did include some information about dissemination of trial results. However, modes of dissemination tended to target researchers and clinicians rather than participants and information on when results would be available was often lacking. The findings highlight the need for further research that includes stakeholder input to identify what information on results summaries participants need at the point of making a decision about trial participation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Beauchamp TL. Principles of Biomedical Ethics, 7th edn. New York; Oxford: Oxford University Press, 2012.
-
- Health Research Authority. Informing participants and seeking consent, 2019. https://www.hra.nhs.uk/planning-and-improving-research/best-practice/inf... [Accessed 20 August 2019].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources