Asymmetric wall ingrowth deposition in Arabidopsis phloem parenchyma transfer cells is tightly associated with sieve elements
- PMID: 35609084
- PMCID: PMC9467654
- DOI: 10.1093/jxb/erac234
Asymmetric wall ingrowth deposition in Arabidopsis phloem parenchyma transfer cells is tightly associated with sieve elements
Abstract
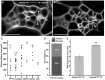
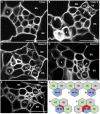
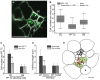
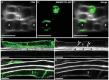
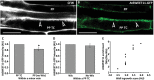
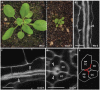
In Arabidopsis, polarized deposition of wall ingrowths in phloem parenchyma (PP) transfer cells (TCs) occurs adjacent to cells of the sieve element/companion cell (SE/CC) complex. However, the spatial relationships between these different cell types in minor veins, where phloem loading occurs, are poorly understood. PP TC development and wall ingrowth localization were compared with those of other phloem cells in leaves of Col-0 and the transgenic lines AtSUC2::AtSTP9-GFP (green fluorescent protein) and AtSWEET11::AtSWEET11-GFP that identify CCs and PP cells, respectively. The development of PP TCs in minor veins, indicated by deposition of wall ingrowths, proceeded basipetally in leaves. However, not all PP cells develop wall ingrowths, and higher levels of deposition occur in abaxial- compared with adaxial-positioned PP TCs. Furthermore, the deposition of wall ingrowths was exclusively initiated on and preferentially covered the PP TC/SE interface, rather than the PP TC/CC interface, and only occurred in PP cells that were adjacent to SEs. Collectively, these results demonstrate a tight association between SEs and wall ingrowth deposition in PP TCs and suggest the existence of two subtypes of PP cells in leaf minor veins. Compared with PP cells, PP TCs showed more abundant accumulation of AtSWEET11-GFP, indicating functional differences in phloem loading between PP and PP TCs.
Keywords: Arabidopsis; AtSUC2; AtSWEET11; companion cells; minor vein; phloem loading; phloem parenchyma transfer cell; sieve elements; wall ingrowths.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures
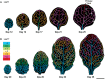

References
-
- Amiard V, Demmig-Adams B, Mueh KE, Turgeon R, Combs AF, Adams WW 3rd. 2007. Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytologist 173, 722–731. - PubMed
-
- Cayla T, Le Hir R, Dinant S.. 2019. Live-cell imaging of fluorescently tagged phloem proteins with confocal microscopy. Methods in Molecular Biology 2014, 95–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

