ZC3HC1 is a structural element of the nuclear basket effecting interlinkage of TPR polypeptides
- PMID: 35609216
- PMCID: PMC9582634
- DOI: 10.1091/mbc.E22-02-0037
ZC3HC1 is a structural element of the nuclear basket effecting interlinkage of TPR polypeptides
Abstract
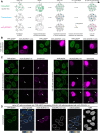
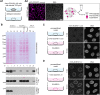
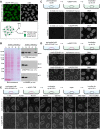
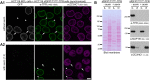
The nuclear basket (NB), anchored to the nuclear pore complex (NPC), is commonly looked upon as a structure built solely of protein TPR polypeptides, the latter thus regarded as the NB's only scaffold-forming components. In the current study, we report ZC3HC1 as a second structural element of the NB. Recently described as an NB-appended protein omnipresent in vertebrates, we now show that ZC3HC1, both in vivo and in vitro, enables in a stepwise manner the recruitment of TPR subpopulations to the NB and their linkage to already NPC-anchored TPR polypeptides. We further demonstrate that the degron-mediated rapid elimination of ZC3HC1 results in the prompt detachment of the ZC3HC1-appended TPR polypeptides from the NB and their release into the nucleoplasm, underscoring the role of ZC3HC1 as a natural structural element of the NB. Finally, we show that ZC3HC1 can keep TPR polypeptides positioned and linked to each other even at sites remote from the NB, in line with ZC3HC1 functioning as a protein connecting TPR polypeptides.
Figures

References
-
- Aksenova V, Lee HN, Smith A, Chen S, Bhat P, Iben J, Echeverria C, Fontoura B, Arnaoutov A, Dasso M (2019). Distinct basket nucleoporins roles in nuclear pore function and gene expression: Tpr is an integral component of the TREX-2 mRNA export pathway. BioRxiv, https://doi .org./10.1101/685263. - DOI
-
- Ashkenazy-Titelman A, Shav-Tal Y, Kehlenbach RH (2020). Into the basket and beyond: the journey of mRNA through the nuclear pore complex. Biochem J 477, 23–44. - PubMed
-
- Bassermann F, von Klitzing C, Illert AL, Münch S, Morris SW, Pagano M, Peschel C, Duyster J, Klitzing C, Illert AL, et al. (2007). Multisite phosphorylation of nuclear interaction partner of ALK (NIPA) at G2/M involves cyclin B1/Cdk1. J Biol Chem 282, 15965–15972. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

