Uncommon Benign Neoplasms and Pseudotumors of the Liver
- PMID: 35609332
- PMCID: PMC10443935
- DOI: 10.5858/arpa.2021-0539-RA
Uncommon Benign Neoplasms and Pseudotumors of the Liver
Abstract
Context.—: The most common benign hepatic mass-forming lesions often display fairly specific imaging characteristics, whereas less familiar, rarer benign neoplasms and pseudotumors may pose a diagnostic challenge in clinical, radiology, and pathology practice because of either their rarity or their unusual features.
Objective.—: To review a selection of pseudotumors and unusual benign hepatic neoplasms encountered in consultation practices with a focus on nonepithelial tumors.
Data sources.—: Sources include English-language literature and personal experiences.
Conclusions.—: Several benign conditions (namely, segmental atrophy, infections, immunoglobulin G4 [IgG4]-related sclerosing disease, angiomyolipoma, mesenchymal hamartoma, and various vascular lesions) can lead to formation of hepatic masses. Because of their rarity and underrecognition, such lesions are often diagnostically challenging. Awareness of hepatic pseudotumors and various rare hepatic neoplasms and their potential mimics can forestall misdiagnosis and inappropriate management.
© 2023 College of American Pathologists.
Conflict of interest statement
The authors have no relevant financial interest in the products or companies described in this article.
Figures

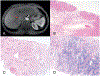



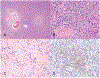

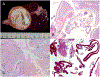

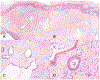


References
-
- Bedada AG, Sreekumaran MI, Azzie G. Segmental atrophy of the liver in a child: case report and review of the literature. S Afr J Child Health. 2019;13(2): 100–101. doi:10.7196/SAJCH.2019.v13i2.1574 - DOI
-
- Assarzadegan N, Estevez-Castro R, Hutchings D, et al. Segmental atrophy (pseudotumor) of the liver: a marker of cardiovascular disease that mimics neoplasia. Mod Pathol. 2020;33(suppl 2):1500.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

