Mitigation of doxorubicin-induced cardiotoxicity with an H2O2-Activated, H2S-Donating hybrid prodrug
- PMID: 35609400
- PMCID: PMC9126844
- DOI: 10.1016/j.redox.2022.102338
Mitigation of doxorubicin-induced cardiotoxicity with an H2O2-Activated, H2S-Donating hybrid prodrug
Abstract
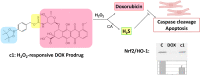
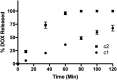
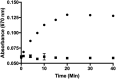
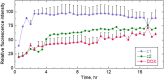
Doxorubicin (DOX) is one of the most effective anticancer agents in clinical oncology. Its continued use, however, is severely limited by its dose-dependent cardiotoxicity which stems, in part, from its overproduction of reactive oxygen species (ROS) and often manifests itself as full-blown cardiomyopathy in patients, years after the cessation of treatment. Therefore, identifying DOX analogs, or prodrugs, with a diminished cardiotoxic profile is highly desirable. Herein, we describe a novel, H2O2-responsive DOX hybrid codrug (mutual prodrug) that has been rationally designed to concurrently liberate hydrogen sulfide (H2S), a purported cardioprotectant with anticancer activity, in an effort to maintain the antitumor effects of DOX while simultaneously reducing its cardiotoxic side effects. Experiments with cardiomyoblast cells in culture demonstrated a rapid accumulation of prodrug into the cells, but diminished apoptotic effects compared with DOX, dependent upon its release of H2S. Cells treated with the prodrug exhibited significantly higher Nrf2 activation relative to DOX-treated cells. Preliminary indications, using a mouse triple-negative breast cancer cell line sensitive to DOX treatment, are that the prodrug maintains considerable toxicity against the tumor-inducing cell line, suggesting significant promise for this prodrug as a cardioprotective chemotherapeutic to replace DOX.
Keywords: Cardiotoxicity; Chemotherapeutic; Doxorubicin; Hydrogen peroxide; Hydrogen sulfide.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: John C. Lukesh reports financial support was provided by National Science Foundation. Leslie B. Poole reports financial support was provided by National Institute of General Medical Sciences. John C. Lukesh and Leslie B. Poole have a provisional patent pending to Wake Forest University.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

