A comprehensive profiling of the immune microenvironment of breast cancer brain metastases
- PMID: 35609559
- PMCID: PMC9713504
- DOI: 10.1093/neuonc/noac136
A comprehensive profiling of the immune microenvironment of breast cancer brain metastases
Abstract
Background: Despite potential clinical implications, the complexity of breast cancer (BC) brain metastases (BM) immune microenvironment is poorly understood. Through multiplex immunofluorescence, we here describe the main features of BCBM immune microenvironment (density and spatial distribution) and evaluate its prognostic impact.
Methods: Sixty BCBM from patients undergoing neurosurgery at three institutions (2003-2018) were comprehensively assessed using two multiplex immunofluorescence panels (CD4, CD8, Granzyme B, FoxP3, CD68, pan-cytokeratin, DAPI; CD3, PD-1, PD-L1, LAG-3, TIM-3, CD163, pan-cytokeratin, DAPI). The prognostic impact of immune subpopulations and cell-to-cell spatial interactions was evaluated.
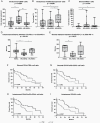
Results: Subtype-related differences in BCBM immune microenvironment and its prognostic impact were observed. While in HR-/HER2- BM and HER2+ BM, higher densities of intra-tumoral CD8+ lymphocytes were associated with significantly longer OS (HR 0.16 and 0.20, respectively), in HR+/HER2- BCBMs a higher CD4+FoxP3+/CD8+ cell ratio in the stroma was associated with worse OS (HR 5.4). Moreover, a higher density of intra-tumoral CD163+ M2-polarized microglia/macrophages in BCBMs was significantly associated with worse OS in HR-/HER2- and HR+/HER2- BCBMs (HR 6.56 and 4.68, respectively), but not in HER2+ BCBMs. In HER2+ BCBMs, multiplex immunofluorescence highlighted a negative prognostic role of PD-1/PD-L1 interaction: patients with a higher percentage of PD-L1+ cells spatially interacting with (within a 20 µm radius) PD-1+ cells presented a significantly worse OS (HR 4.60).
Conclusions: Our results highlight subtype-related differences in BCBM immune microenvironment and identify two potential therapeutic targets, M2 microglia/macrophage polarization in HER2- and PD-1/PD-L1 interaction in HER2+ BCBMs, which warrant future exploration in clinical trials.
Keywords: brain metastases; breast cancer; immune biomarkers; immune microenvironment; multiplex immunofluorescence.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures



References
-
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. - PubMed
-
- Bendell JC, Domchek SM, Burstein HJ, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. - PubMed
-
- Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

