Approaches to Treat Sensorineural Hearing Loss by Hair-Cell Regeneration: The Current State of Therapeutic Developments and Their Potential Impact on Audiological Clinical Practice
- PMID: 35609593
- PMCID: PMC9129918
- DOI: 10.1055/s-0042-1750281
Approaches to Treat Sensorineural Hearing Loss by Hair-Cell Regeneration: The Current State of Therapeutic Developments and Their Potential Impact on Audiological Clinical Practice
Abstract
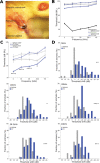
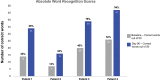
Sensorineural hearing loss (SNHL) is typically a permanent and often progressive condition that is commonly attributed to sensory cell loss. All vertebrates except mammals can regenerate lost sensory cells. Thus, SNHL is currently only treated with hearing aids or cochlear implants. There has been extensive research to understand how regeneration occurs in nonmammals, how hair cells form during development, and what limits regeneration in maturing mammals. These studies motivated efforts to identify therapeutic interventions to regenerate hair cells as a treatment for hearing loss, with a focus on targeting supporting cells to form new sensory hair cells. The approaches include gene therapy and small molecule delivery to the inner ear. At the time of this publication, early-stage clinical trials have been conducted to test targets that have shown evidence of regenerating sensory hair cells in preclinical models. As these potential treatments move closer to a clinical reality, it will be important to understand which therapeutic option is most appropriate for a given population. It is also important to consider which audiological tests should be administered to identify hearing improvement while considering the pharmacokinetics and mechanism of a given approach. Some impacts on audiological practice could include implementing less common audiological measures as standard procedure. As devices are not capable of repairing the damaged underlying biology, hair-cell regeneration treatments could allow patients to benefit more from their devices, move from a cochlear implant candidate to a hearing aid candidate, or move a subject to not needing an assistive device. Here, we describe the background, current state, and future implications of hair-cell regeneration research.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Ashley S. Hinton, Christopher Loose and Will J. McLean are all employees of Frequency Therapeutics.
Figures


References
-
- Blackwell D L, Lucas J W, Clarke T C. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Heal Stat 10. 2014;(260):1–161. - PubMed
-
- World Health Organization (WHO) . Hearing loss due to recreational exposure to loud sounds: a review. 2015.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

