Electron transfer activity of the nanodisc-bound mitochondrial outer membrane protein mitoNEET
- PMID: 35609862
- PMCID: PMC10693299
- DOI: 10.1016/j.freeradbiomed.2022.05.011
Electron transfer activity of the nanodisc-bound mitochondrial outer membrane protein mitoNEET
Abstract
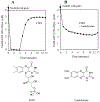
MitoNEET is the first iron-sulfur protein found in mitochondrial outer membrane. Abnormal expression of mitoNEET in cells has been linked to several types of cancer, type II diabetes, and neurodegenerative diseases. Structurally, mitoNEET is anchored to mitochondrial outer membrane via its N-terminal single transmembrane alpha helix. The C-terminal cytosolic domain of mitoNEET binds a [2Fe-2S] cluster via three cysteine and one histidine residues. It has been shown that mitoNEET has a crucial role in energy metabolism, iron homeostasis, and free radical production in cells. However, the exact function of mitoNEET remains elusive. Previously, we reported that the C-terminal soluble domain of mitoNEET has a specific binding site for flavin mononucleotide (FMN) and can transfer electrons from FMNH2 to oxygen or ubiquinone-2 via its [2Fe-2S] cluster. Here we have constructed a hybrid protein using the N-terminal transmembrane domain of Escherichia coli YneM and the C-terminal soluble domain of human mitoNEET and assembled the hybrid protein YneM-mitoNEET into phospholipid nanodiscs. The results show that the [2Fe-S] clusters in the nanodisc-bound YneM-mitoNEET can be rapidly reduced by FMNH2 which is reduced by flavin reductase using NADH as the electron donor. Addition of lumichrome, a FMN analog, effectively inhibits the FMNH2-mediated reduction of the [2Fe-2S] clusters in the nanodisc-bound YneM-mitoNEET. The reduced [2Fe-2S] clusters in the nanodisc-bound YneM-mitoNEET are quickly oxidized by oxygen under aerobic conditions or by ubiquinone-10 in the nanodiscs under anaerobic conditions. Because NADH oxidation is required for cellular glycolytic activity, we propose that the mitochondrial outer membrane protein mitoNEET may promote glycolysis by transferring electrons from FMNH2 to oxygen or ubiquinone-10 in mitochondria.
Keywords: Electron transfer activity; FMN; Mitochondria; NADH; Nanodisc; mitoNEET.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

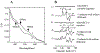

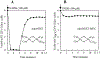
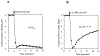

References
-
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR, Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe, Am. J. Physiol. Endocrinol. Metab 286(2) (2004) E252–E260. - PubMed
-
- Tamir S, Paddock ML, Darash-Yahana-Baram M, Holt SH, Sohn YS, Agranat L, Michaeli D, Stofleth JT, Lipper CH, Morcos F, Cabantchik IZ, Onuchic JN, Jennings PA, Mittler R, Nechushtai R, Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease, Biochim. Biophys. Acta 1853(6) (2015) 1294–315. - PubMed
-
- Bai F, Morcos F, Sohn YS, Darash-Yahana M, Rezende CO, Lipper CH, Paddock ML, Song L, Luo Y, Holt SH, Tamir S, Theodorakis EA, Jennings PA, Onuchic JN, Mittler R, Nechushtai R, The Fe-S cluster-containing NEET proteins mitoNEET and NAF-1 as chemotherapeutic targets in breast cancer, Proc. Natl. Acad. Sci. U. S. A 112(12) (2015) 3698–703. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

