Carbene Complexes of Neptunium
- PMID: 35609882
- PMCID: PMC9490846
- DOI: 10.1021/jacs.2c02152
Carbene Complexes of Neptunium
Abstract
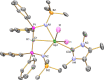
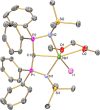
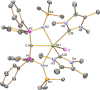
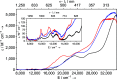
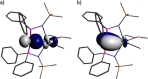
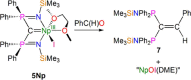
Since the advent of organotransuranium chemistry six decades ago, structurally verified complexes remain restricted to π-bonded carbocycle and σ-bonded hydrocarbyl derivatives. Thus, transuranium-carbon multiple or dative bonds are yet to be reported. Here, utilizing diphosphoniomethanide precursors we report the synthesis and characterization of transuranium-carbene derivatives, namely, diphosphonio-alkylidene- and N-heterocyclic carbene-neptunium(III) complexes that exhibit polarized-covalent σ2π2 multiple and dative σ2 single transuranium-carbon bond interactions, respectively. The reaction of [NpIIII3(THF)4] with [Rb(BIPMTMSH)] (BIPMTMSH = {HC(PPh2NSiMe3)2}1-) affords [(BIPMTMSH)NpIII(I)2(THF)] (3Np) in situ, and subsequent treatment with the N-heterocyclic carbene {C(NMeCMe)2} (IMe4) allows isolation of [(BIPMTMSH)NpIII(I)2(IMe4)] (4Np). Separate treatment of in situ prepared 3Np with benzyl potassium in 1,2-dimethoxyethane (DME) affords [(BIPMTMS)NpIII(I)(DME)] (5Np, BIPMTMS = {C(PPh2NSiMe3)2}2-). Analogously, addition of benzyl potassium and IMe4 to 4Np gives [(BIPMTMS)NpIII(I)(IMe4)2] (6Np). The synthesis of 3Np-6Np was facilitated by adopting a scaled-down prechoreographed approach using cerium synthetic surrogates. The thorium(III) and uranium(III) analogues of these neptunium(III) complexes are currently unavailable, meaning that the synthesis of 4Np-6Np provides an example of experimental grounding of 5f- vs 5f- and 5f- vs 4f-element bonding and reactivity comparisons being led by nonaqueous transuranium chemistry rather than thorium and uranium congeners. Computational analysis suggests that these NpIII═C bonds are more covalent than UIII═C, CeIII═C, and PmIII═C congeners but comparable to analogous UIV═C bonds in terms of bond orders and total metal contributions to the M═C bonds. A preliminary assessment of NpIII═C reactivity has introduced multiple bond metathesis to transuranium chemistry, extending the range of known metallo-Wittig reactions to encompass actinide oxidation states III-VI.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

