Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study
- PMID: 35609888
- PMCID: PMC9127435
- DOI: 10.1136/bmj-2022-071113
Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study
Abstract
Objective: To examine the relative effectiveness of a fourth dose of the Pfizer-BioNTech mRNA (BNT162b2) vaccine compared with three vaccine doses over the span of 10 weeks.
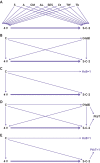
Design: Retrospective, test negative, case-control study, with a matched analysis and an unmatched multiple tests analysis.
Setting: Nationally centralised database of Maccabi Healthcare Services, an Israeli national health fund for 2.5 million people; from 10 January 2022 (seven days after the fourth dose was first given to eligible individuals) to 13 March 2022, an omicron dominant period in Israel.
Participants: 97 499 Maccabi Healthcare Services members aged 60 years and older, who were eligible to receive a fourth vaccine dose and obtained at least one polymerase chain reaction (PCR) test during the study.
Main outcome measures: Breakthrough SARS-CoV-2 infection, defined as a positive PCR test performed seven or more days after inoculation with the BNT162b2 vaccine; and breakthrough SARS-CoV-2 infection resulting in severe covid-19 disease, defined as hospital admission or death related to covid-19.
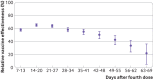
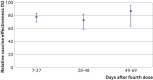
Results: 27 876 participants received the fourth BNT162b2 vaccine dose and 69 623 received three doses only. Of 106 participants who died during the follow-up period, 77 had had their third doses only and 23 had had their fourth doses during the first three weeks after inoculation. In the first three weeks, a fourth dose provided additional protection against both SARS-CoV-2 infection and severe disease relative to three doses of the vaccine. However, relative vaccine effectiveness against infection quickly decreased over time, peaking during the third week at 65.1% (95% confidence interval 63.0% to 67.1%) and falling to 22.0% (4.9% to 36.1%) by the end of the 10 week follow-up period. Unlike relative effectiveness against SARS-CoV-2 infection, the relative effectiveness of a fourth dose against severe covid-19 was maintained at a high level (>72%) throughout follow-up. However, severe disease was a relatively rare event, occurring in <1% of study participants who received four doses or three doses only.
Conclusions: A fourth dose of the BNT162b2 vaccine appears to have provided additional protection against both SARS-CoV-2 infection and severe covid-19 disease relative to three vaccine doses. However, relative effectiveness of the fourth dose against infection appears to wane sooner than that of the third dose.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support for VEP from the National Institutes of Health for the submitted work; VEP has received reimbursement from Merck and Pfizer for travel to scientific input engagements unrelated to the topic of this manuscript and is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee; all other authors declare they have no conflict of interest.
Figures
References
-
- COVID-19 in Israel dashboard. Isr. Minist. Heal. https://datadashboard.health.gov.il/COVID-19/general (accessed 15 Apr 2022).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
