First and second doses of Covishield vaccine provided high level of protection against SARS-CoV-2 infection in highly transmissible settings: results from a prospective cohort of participants residing in congregate facilities in India
- PMID: 35609920
- PMCID: PMC9130647
- DOI: 10.1136/bmjgh-2021-008271
First and second doses of Covishield vaccine provided high level of protection against SARS-CoV-2 infection in highly transmissible settings: results from a prospective cohort of participants residing in congregate facilities in India
Abstract
Objectives: This study aimed to determine the effectiveness of Covishield vaccine among residents of congregate residential facilities.
Design: A prospective cohort study in congregate residential facilities.
Setting: Dharamshala, Himachal Pradesh, India, from December 2020 to July 2021.
Participants: Residents of all ages in seven facilities-three monasteries, two old age homes and two learning centres-were enrolled.
Exposures: First and second doses of Covishield vaccine against SARS-CoV-2 infection.
Main outcomes measures: Primary outcome was development of COVID-19. Secondary outcome was unfavourable outcomes, defined as a composite of shortness of breath, hospitalisation or death. Vaccine effectiveness (%) was calculated as (1-HR)×100.
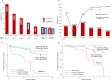
Results: There were 1114 residents (median age 31 years) participating in the study, 82% males. Twenty-eight per cent (n=308/1114) were unvaccinated, 50% (n=554/1114) had received one dose and 23% (n=252/1114) had received two doses of Covishield. The point prevalence of COVID-19 for the facilities ranged from 11% to 57%. Incidence rates (95% CI) of COVID-19 were 76 (63 to 90)/1000 person-months in the unvaccinated, 25 (18 to 35)/1000 person-months in recipients of one dose and 9 (4 to 19)/1000 person-months in recipients of two doses. The effectiveness of first and second doses of Covishield were 71% (adjusted HR (aHR) 0.29; 95% CI 0.18 to 0.46; p<0.001) and 80% (aHR 0.20; 95% CI 0.09 to 0.44; p<0.001), respectively, against SARS-CoV-2 infection and 86% (aHR 0.24; 95% CI 0.07 to 0.82; p=0.023) and 99% (aHR 0.01; 95% CI 0.002 to 0.10; p<0.001), respectively, against unfavourable outcome. The effectiveness was higher after 14 days of receiving the first and second doses, 93% and 98%, respectively. Risk of infection was higher in persons with chronic hepatitis B (aHR 1.78; p=0.034) and previous history of tuberculosis (aHR 1.62; p=0.047).
Conclusion: Covishield was effective in preventing SARS-CoV-2 infection and reducing disease severity in highly transmissible settings during the second wave of the pandemic driven by the Delta variant.
Keywords: COVID-19; Epidemiology; Public Health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effectiveness of ChAdOx1 nCoV-19 and BBIBP-CorV vaccines against COVID-19-associated hospitalisation and death in the Seychelles infected adult population.PLoS One. 2024 Apr 5;19(4):e0299747. doi: 10.1371/journal.pone.0299747. eCollection 2024. PLoS One. 2024. PMID: 38578809 Free PMC article.
-
Effectiveness of BBV152/Covaxin and AZD1222/Covishield vaccines against severe COVID-19 and B.1.617.2/Delta variant in India, 2021: a multi-centric hospital-based case-control study.Int J Infect Dis. 2022 Sep;122:693-702. doi: 10.1016/j.ijid.2022.07.033. Epub 2022 Jul 16. Int J Infect Dis. 2022. PMID: 35843496 Free PMC article.
-
Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021.MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1163-1166. doi: 10.15585/mmwr.mm7034e3. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34437519 Free PMC article.
-
Covid-19 Vaccines Available in India.Comb Chem High Throughput Screen. 2022;25(14):2391-2397. doi: 10.2174/1386207325666220315115953. Comb Chem High Throughput Screen. 2022. PMID: 35293291 Review.
-
Immunogenic and reactogenic efficacy of Covaxin and Covishield: a comparative review.Immunol Res. 2022 Jun;70(3):289-315. doi: 10.1007/s12026-022-09265-0. Epub 2022 Feb 22. Immunol Res. 2022. PMID: 35192185 Free PMC article. Review.
Cited by
-
SARS-CoV-2 breakthrough infections during the second wave of COVID-19 at Pune, India.Front Public Health. 2023 Jan 12;10:1040012. doi: 10.3389/fpubh.2022.1040012. eCollection 2022. Front Public Health. 2023. PMID: 36711329 Free PMC article.
-
Effectiveness of ChAdOx1 nCoV-19 and BBIBP-CorV vaccines against COVID-19-associated hospitalisation and death in the Seychelles infected adult population.PLoS One. 2024 Apr 5;19(4):e0299747. doi: 10.1371/journal.pone.0299747. eCollection 2024. PLoS One. 2024. PMID: 38578809 Free PMC article.
-
COVID-19 vaccines and a perspective on Africa.Trends Immunol. 2023 Mar;44(3):172-187. doi: 10.1016/j.it.2023.01.005. Epub 2023 Jan 11. Trends Immunol. 2023. PMID: 36709083 Free PMC article. Review.
-
Immunogenicity of two COVID-19 vaccines used in India: An observational cohort study in health care workers from a tertiary care hospital.Front Immunol. 2022 Sep 23;13:928501. doi: 10.3389/fimmu.2022.928501. eCollection 2022. Front Immunol. 2022. PMID: 36211366 Free PMC article.
-
Antibody titres in fully vaccinated healthcare workers with and without breakthrough infection during the Delta and Omicron waves.J Family Med Prim Care. 2023 Jul;12(7):1298-1302. doi: 10.4103/jfmpc.jfmpc_1809_22. Epub 2023 Jul 14. J Family Med Prim Care. 2023. PMID: 37649769 Free PMC article.
References
-
- Self WH, Tenforde MW, Rhoads JP, et al. . Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1337–43. 10.15585/mmwr.mm7038e1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
