Comparing genome sequencing technologies to improve rare disease diagnostics: a protocol for the evaluation of a pilot project, Genome-wide Sequencing Ontario
- PMID: 35609929
- PMCID: PMC9259466
- DOI: 10.9778/cmajo.20210272
Comparing genome sequencing technologies to improve rare disease diagnostics: a protocol for the evaluation of a pilot project, Genome-wide Sequencing Ontario
Abstract
Background: Genome-wide sequencing has emerged as a promising strategy for the timely diagnosis of rare diseases, but it is not yet available as a clinical test performed in Canadian diagnostic laboratories. We describe the protocol for evaluating a 2-year pilot project, Genome-wide Sequencing Ontario, to offer high-quality clinical genome-wide sequencing in Ontario, Canada.
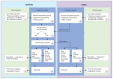
Methods: The Genome-wide Sequencing Ontario protocol was codesigned by the Ontario Ministry of Health, the Hospital for Sick Children in Toronto and the Children's Hospital of Eastern Ontario in Ottawa. Enrolment of a prospective cohort of patients began on Apr. 1, 2021. Eligible cases with blood samples available for the index case and both parents (i.e., trios) are randomized to receive exome sequencing or genome sequencing. We will collect patient-level data and ascertain costs associated with the laboratory workflow for exome sequencing and genome sequencing. We will compare point estimates for the diagnostic utility and timeliness of exome sequencing and genome sequencing, and we will determine an incremental cost-effectiveness ratio (expressed as the incremental cost of genome sequencing versus exome sequencing per additional patient with a causal variant detected).
Interpretation: Findings from this work will provide robust evidence for the diagnostic utility, cost-effectiveness and timeliness of exome sequencing and genome sequencing, and will be disseminated via academic publications and policy briefs. Findings will inform provincial and cross-provincial policy related to the long-term organization, delivery and reimbursement of clinical-grade genome diagnostics for rare disease.
© 2022 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests: The authorship group has received funding from the Ontario Ministry of Health and Genome Canada to examine the implementation of genome-wide sequencing in Ontario. Dimitri Stavropoulos has equity in PhenoTips. Wendy Ungar chairs the Ontario Genetics Advisory Committee, is a member of the Ontario Health Technology Assessment Committee, and receives funding from the Pharmaceutical Research and Manufacturers of America Foundation and honoraria from the Taiwan Center for Drug Evaluation. Robin Hayeems is a member of the Ontario Genetics Advisory Committee. Robin Hayeems, Martin Somerville and Kym Boycott are members of the Provincial Genetics Advisory Committee.
Figures
References
-
- Boycott KM, Vanstone M, Bulman DE, et al. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14:681–91. - PubMed
-
- Stark Z, Tan TY, Chong B, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous