Signal Transduction Network Principles Underlying Bacterial Collective Behaviors
- PMID: 35609948
- PMCID: PMC9463083
- DOI: 10.1146/annurev-micro-042922-122020
Signal Transduction Network Principles Underlying Bacterial Collective Behaviors
Abstract
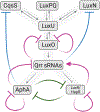
Bacteria orchestrate collective behaviors and accomplish feats that would be unsuccessful if carried out by a lone bacterium. Processes undertaken by groups of bacteria include bioluminescence, biofilm formation, virulence factor production, and release of public goods that are shared by the community. Collective behaviors are controlled by signal transduction networks that integrate sensory information and transduce the information internally. Here, we discuss network features and mechanisms that, even in the face of dramatically changing environments, drive precise execution of bacterial group behaviors. We focus on representative quorum-sensing and second-messenger cyclic dimeric GMP (c-di-GMP) signal relays. We highlight ligand specificity versus sensitivity, how small-molecule ligands drive discrimination of kin versus nonkin, signal integration mechanisms, single-input sensory systems versus coincidence detectors, and tuning of input-output dynamics via feedback regulation. We summarize how different features of signal transduction systems allow groups of bacteria to successfully interpret and collectively react to dynamically changing environments.
Keywords: bacterial group behavior; c-di-GMP; feedback; quorum sensing; sensitivity; signal transduction; specificity.
Figures
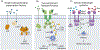


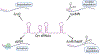
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

