Neuropathologic Features of Antemortem Atrophy-Based Subtypes of Alzheimer Disease
- PMID: 35609990
- PMCID: PMC9421777
- DOI: 10.1212/WNL.0000000000200573
Neuropathologic Features of Antemortem Atrophy-Based Subtypes of Alzheimer Disease
Erratum in
-
Neuropathologic Features of Antemortem Atrophy-Based Subtypes of Alzheimer Disease.Neurology. 2023 Jan 17;100(3):165. doi: 10.1212/WNL.0000000000201298. Epub 2022 Sep 30. Neurology. 2023. PMID: 36180246 Free PMC article. No abstract available.
Abstract
Background and objectives: To investigate whether antemortem MRI-based atrophy subtypes of Alzheimer disease (AD) differ in neuropathologic features and comorbid non-AD pathologies at postmortem.
Methods: From the Alzheimer's Disease Neuroimaging Initiative cohort, we included individuals with antemortem MRI evaluating brain atrophy within 2 years before death, antemortem diagnosis of AD dementia/mild cognitive impairment, and postmortem-confirmed AD neuropathologic change. Antemortem atrophy subtypes were modeled as continuous phenomena based on a recent conceptual framework: typicality (spanning limbic-predominant AD to hippocampal-sparing AD) and severity (spanning typical AD to minimal atrophy AD). Postmortem neuropathologic evaluation included AD hallmarks, β-amyloid, and tau as well as non-AD pathologies, alpha-synuclein and TAR DNA-binding protein 43 (TDP-43). We also investigated the overall concomitance across these pathologies. Partial correlations assessed the associations between antemortem atrophy subtypes and postmortem neuropathologic outcomes.
Results: In 31 individuals (26 AD dementia/5 mild cognitive impairment, mean age = 80 years, 26% females), antemortem typicality was significantly negatively associated with neuropathologic features, including β-amyloid (rho = -0.39 overall), tau (rho = -0.38 regionally), alpha-synuclein (rho = -0.39 regionally), TDP-43 (rho = -0.49 overall), and concomitance of pathologies (rho = -0.59 regionally). Limbic-predominant AD was associated with higher Thal phase, neuritic plaque density, and presence of TDP-43 compared with hippocampal-sparing AD. Regionally, limbic-predominant AD showed a higher presence of tau and alpha-synuclein pathologies in medial temporal structures, a higher presence of TDP-43, and concomitance of pathologies subcortically/cortically compared with hippocampal-sparing AD. Antemortem severity was significantly negatively associated with concomitance of pathologies (rho = -0.43 regionally), such that typical AD showed higher concomitance of pathologies than minimal atrophy AD.
Discussion: We provide a direct antemortem-to-postmortem validation, highlighting the importance of understanding atrophy-based heterogeneity in AD relative to AD and non-AD pathologies. We suggest that (1) typicality and severity in atrophy reflect differential aspects of susceptibility of the brain to AD and non-AD pathologies; and (2) limbic-predominant AD and typical AD subtypes share similar biological pathways, making them more vulnerable to AD and non-AD pathologies compared with hippocampal-sparing AD, which may follow a different biological pathway. Our findings provide a deeper understanding of associations of atrophy subtypes in AD with different pathologies, enhancing the prevailing knowledge of biological heterogeneity in AD and could contribute toward tracking disease progression and designing clinical trials in the future.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

 ; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =
; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =  , whereby higher values correspond to lower severity. AD = Alzheimer disease; ADNC = AD neuropathologic change; ALB = amygdala Lewy bodies; aMCI = amnestic mild cognitive impairment; DLB = dementia with Lewy bodies; HS = hippocampal-sparing AD; HSc = hippocampal sclerosis; ICH = intracerebral hemorrhage; LP = limbic-predominant AD; MA = minimal atrophy AD; RID = assigned individual ID in the AD Neuroimaging Initiative dataset; SAL = subcortical arteriosclerotic leukoencephalopathy; SDH = subdural hemorrhage; TAD = typical AD; TDP-MTL = TAR DNA-binding protein in the medial temporal lobe.
, whereby higher values correspond to lower severity. AD = Alzheimer disease; ADNC = AD neuropathologic change; ALB = amygdala Lewy bodies; aMCI = amnestic mild cognitive impairment; DLB = dementia with Lewy bodies; HS = hippocampal-sparing AD; HSc = hippocampal sclerosis; ICH = intracerebral hemorrhage; LP = limbic-predominant AD; MA = minimal atrophy AD; RID = assigned individual ID in the AD Neuroimaging Initiative dataset; SAL = subcortical arteriosclerotic leukoencephalopathy; SDH = subdural hemorrhage; TAD = typical AD; TDP-MTL = TAR DNA-binding protein in the medial temporal lobe.
 ; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =
; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =  , whereby higher values correspond to lower severity. AD = Alzheimer disease; ADNC = AD neuropathologic change.
, whereby higher values correspond to lower severity. AD = Alzheimer disease; ADNC = AD neuropathologic change.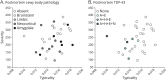
 ; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =
; all plots show antemortem MRI-based severity on the vertical scale, proxied by the Global Brain Atrophy Index =  , whereby higher values correspond to lower severity. A + E = TDP-43 immunoreactive inclusions are present in the amygdala and entorhinal/inferior temporal cortex; A + H + E + N = TDP-43 immunoreactive inclusions are present in the amygdala, hippocampus, entorhinal/inferior temporal cortex, and neocortex; A + H + E = TDP-43 immunoreactive inclusions are present in the amygdala, hippocampus, and entorhinal/inferior temporal cortex; AD = Alzheimer disease; TDP-43 = TAR DNA-binding protein 43.
, whereby higher values correspond to lower severity. A + E = TDP-43 immunoreactive inclusions are present in the amygdala and entorhinal/inferior temporal cortex; A + H + E + N = TDP-43 immunoreactive inclusions are present in the amygdala, hippocampus, entorhinal/inferior temporal cortex, and neocortex; A + H + E = TDP-43 immunoreactive inclusions are present in the amygdala, hippocampus, and entorhinal/inferior temporal cortex; AD = Alzheimer disease; TDP-43 = TAR DNA-binding protein 43.

References
-
- Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203-212. - PubMed
