Effects of Short-Term Potassium Chloride Supplementation in Patients with CKD
- PMID: 35609996
- PMCID: PMC9529195
- DOI: 10.1681/ASN.2022020147
Effects of Short-Term Potassium Chloride Supplementation in Patients with CKD
Abstract
Background: Observational studies suggest that adequate dietary potassium intake (90-120 mmol/day) may be renoprotective, but the effects of increasing dietary potassium and the risk of hyperkalemia are unknown.
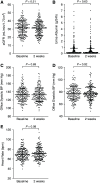
Methods: This is a prespecified analysis of the run-in phase of a clinical trial in which 191 patients (age 68±11 years, 74% males, 86% European ancestry, eGFR 31±9 ml/min per 1.73 m2, 83% renin-angiotensin system inhibitors, 38% diabetes) were treated with 40 mmol potassium chloride (KCl) per day for 2 weeks.
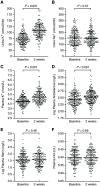
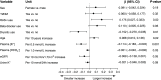
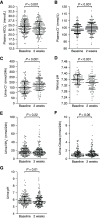
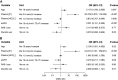
Results: KCl supplementation significantly increased urinary potassium excretion (72±24 to 107±29 mmol/day), plasma potassium (4.3±0.5 to 4.7±0.6 mmol/L), and plasma aldosterone (281 [198-431] to 351 [241-494] ng/L), but had no significant effect on urinary sodium excretion, plasma renin, BP, eGFR, or albuminuria. Furthermore, KCl supplementation increased plasma chloride (104±3 to 105±4 mmol/L) and reduced plasma bicarbonate (24.5±3.4 to 23.7±3.5 mmol/L) and urine pH (all P<0.001), but did not change urinary ammonium excretion. In total, 21 participants (11%) developed hyperkalemia (plasma potassium 5.9±0.4 mmol/L). They were older and had higher baseline plasma potassium.
Conclusions: In patients with CKD stage G3b-4, increasing dietary potassium intake to recommended levels with potassium chloride supplementation raises plasma potassium by 0.4 mmol/L. This may result in hyperkalemia in older patients or those with higher baseline plasma potassium. Longer-term studies should address whether cardiorenal protection outweighs the risk of hyperkalemia.Clinical trial number: NCT03253172.
Keywords: acidosis; aldosterone; chronic kidney disease; clinical trial; dietary supplements; electrolytes; hypertension; potassium chloride.
Copyright © 2022 by the American Society of Nephrology.
Figures

Comment in
-
The Highs and Lows of Potassium Intake in CKD-Does One Size Fit All?J Am Soc Nephrol. 2022 Sep;33(9):1638-1640. doi: 10.1681/ASN.2022070743. Epub 2022 Jul 19. J Am Soc Nephrol. 2022. PMID: 36630492 Free PMC article. No abstract available.
References
-
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 - PubMed
-
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 - PubMed
-
- Carrero JJ, González-Ortiz A, Avesani CM, Bakker SJL, Bellizzi V, Chauveau P, et al. : Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol 16: 525–542, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

